Net Ionic Equation For Acid Base Reaction
Muz Play
Mar 22, 2025 · 6 min read
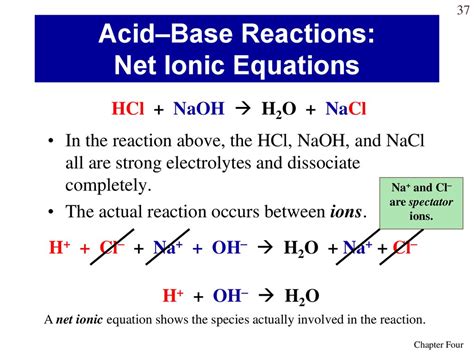
Table of Contents
Net Ionic Equations for Acid-Base Reactions: A Comprehensive Guide
Understanding net ionic equations is crucial for mastering chemistry, especially when dealing with acid-base reactions. This comprehensive guide will delve into the intricacies of net ionic equations, explaining their significance, how to write them, and exploring various examples to solidify your understanding. We'll also touch upon the nuances of strong and weak acids and bases, and how their behavior impacts the net ionic equation.
What is a Net Ionic Equation?
A net ionic equation shows only the species that directly participate in a chemical reaction. It omits spectator ions, which are ions that are present in the reaction mixture but do not undergo any chemical change. In essence, it's a simplified representation of the overall reaction, focusing solely on the essential chemical transformation. This simplification helps us to understand the fundamental chemistry occurring without the clutter of extraneous ions.
Contrast with Molecular and Complete Ionic Equations:
Before we dive deeper, let's clarify the differences between three types of equations:
-
Molecular Equation: This equation represents the reactants and products in their undissociated forms, as if they were whole molecules. It doesn't explicitly show the ions involved.
-
Complete Ionic Equation: This equation shows all the ions present in the solution, both those that participate in the reaction and those that remain unchanged (spectator ions).
-
Net Ionic Equation: As discussed, this equation displays only the ions that undergo a chemical change, effectively removing the spectator ions.
Writing Net Ionic Equations for Acid-Base Reactions
Acid-base reactions, also known as neutralization reactions, involve the reaction between an acid and a base, typically resulting in the formation of water and a salt. To write a net ionic equation for an acid-base reaction, follow these steps:
-
Write the balanced molecular equation: Begin by writing the balanced chemical equation using the chemical formulas of the reactants and products.
-
Write the complete ionic equation: Break down all strong electrolytes (strong acids, strong bases, and soluble salts) into their constituent ions. Weak electrolytes (weak acids and weak bases) remain in their molecular form.
-
Identify and cancel out spectator ions: Spectator ions are present on both the reactant and product sides of the complete ionic equation. These ions are unchanged during the reaction and can be canceled out.
-
Write the net ionic equation: The remaining ions after canceling out the spectator ions constitute the net ionic equation. It should be balanced in terms of both mass and charge.
Examples of Net Ionic Equations for Acid-Base Reactions
Let's illustrate the process with several examples, highlighting the differences between reactions involving strong and weak electrolytes.
Example 1: Reaction of a Strong Acid and a Strong Base
Consider the reaction between hydrochloric acid (HCl), a strong acid, and sodium hydroxide (NaOH), a strong base:
-
Molecular Equation: HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
-
Complete Ionic Equation: H⁺(aq) + Cl⁻(aq) + Na⁺(aq) + OH⁻(aq) → Na⁺(aq) + Cl⁻(aq) + H₂O(l)
-
Identify and cancel spectator ions: Na⁺(aq) and Cl⁻(aq) are spectator ions.
-
Net Ionic Equation: H⁺(aq) + OH⁻(aq) → H₂O(l)
This is the quintessential net ionic equation for the reaction of a strong acid and a strong base. It shows the combination of hydrogen ions and hydroxide ions to form water. This equation is consistent across all reactions involving strong acids and strong bases.
Example 2: Reaction of a Weak Acid and a Strong Base
Let's examine the reaction between acetic acid (CH₃COOH), a weak acid, and sodium hydroxide (NaOH), a strong base:
-
Molecular Equation: CH₃COOH(aq) + NaOH(aq) → CH₃COONa(aq) + H₂O(l)
-
Complete Ionic Equation: CH₃COOH(aq) + Na⁺(aq) + OH⁻(aq) → CH₃COO⁻(aq) + Na⁺(aq) + H₂O(l)
-
Identify and cancel spectator ions: Na⁺(aq) is the spectator ion.
-
Net Ionic Equation: CH₃COOH(aq) + OH⁻(aq) → CH₃COO⁻(aq) + H₂O(l)
Notice that acetic acid, being a weak acid, does not dissociate completely into ions. Therefore, it remains in its molecular form in the net ionic equation.
Example 3: Reaction of a Strong Acid and a Weak Base
Consider the reaction between hydrochloric acid (HCl), a strong acid, and ammonia (NH₃), a weak base:
-
Molecular Equation: HCl(aq) + NH₃(aq) → NH₄Cl(aq)
-
Complete Ionic Equation: H⁺(aq) + Cl⁻(aq) + NH₃(aq) → NH₄⁺(aq) + Cl⁻(aq)
-
Identify and cancel spectator ions: Cl⁻(aq) is the spectator ion.
-
Net Ionic Equation: H⁺(aq) + NH₃(aq) → NH₄⁺(aq)
In this case, ammonia, a weak base, accepts a proton from the strong acid, forming the ammonium ion.
Example 4: Polyprotic Acids
Polyprotic acids, like sulfuric acid (H₂SO₄), can donate more than one proton. The net ionic equation will depend on the stoichiometry and the strength of the acid. For instance, the first ionization of sulfuric acid is complete, while the second is partial.
Example 5: Reactions with Insoluble Salts
If a precipitate forms during the acid-base reaction, the net ionic equation will reflect the formation of the solid. For example, consider the reaction between sulfuric acid and barium hydroxide:
-
Molecular Equation: H₂SO₄(aq) + Ba(OH)₂(aq) → BaSO₄(s) + 2H₂O(l)
-
Complete Ionic Equation: 2H⁺(aq) + SO₄²⁻(aq) + Ba²⁺(aq) + 2OH⁻(aq) → BaSO₄(s) + 2H₂O(l)
-
Net Ionic Equation: 2H⁺(aq) + SO₄²⁻(aq) + Ba²⁺(aq) + 2OH⁻(aq) → BaSO₄(s) + 2H₂O(l)
In this case, there are no spectator ions because barium sulfate is insoluble and precipitates out of the solution.
Significance of Net Ionic Equations
Net ionic equations offer several crucial advantages in understanding chemical reactions:
-
Simplified Representation: They provide a clear and concise picture of the essential chemical changes occurring, eliminating the distraction of spectator ions.
-
Focus on the Chemistry: They highlight the fundamental chemical processes involved, facilitating a better understanding of reaction mechanisms.
-
Predicting Reactions: They can be used to predict the products of acid-base reactions and other ionic reactions.
-
Stoichiometry Calculations: They simplify stoichiometry calculations by focusing only on the reacting species.
-
Understanding Equilibria: They are essential for studying acid-base equilibria and determining equilibrium constants.
Strong vs. Weak Acids and Bases: Impact on Net Ionic Equations
The strength of an acid or base significantly influences its behavior and, consequently, its representation in a net ionic equation.
-
Strong Acids/Bases: Completely dissociate in aqueous solutions, meaning they exist entirely as ions. They are always shown as ions in complete and net ionic equations. Examples include HCl, HNO₃, H₂SO₄ (first proton only), NaOH, KOH.
-
Weak Acids/Bases: Partially dissociate in aqueous solutions, meaning they exist as a mixture of ions and undissociated molecules. They are typically shown in their molecular form in net ionic equations unless they are involved in a reaction that leads to complete consumption of the weak acid/base. Examples include CH₃COOH, HF, NH₃.
Understanding this distinction is critical for accurately writing net ionic equations for acid-base reactions involving weak electrolytes.
Conclusion
Net ionic equations are a fundamental tool for understanding and representing chemical reactions, particularly acid-base reactions. By following the systematic steps outlined above and considering the nature of the acids and bases involved, you can confidently write and interpret net ionic equations, thereby deepening your comprehension of chemical processes. Remember to always identify and cancel spectator ions to obtain the simplified and meaningful net ionic equation. Mastering this skill is crucial for success in chemistry. Continue practicing with various examples to enhance your understanding and proficiency in writing net ionic equations.
Latest Posts
Latest Posts
-
The Mole And Avogadros Number Worksheet Answers
Mar 23, 2025
-
What Are The 7 Evidence Of Evolution
Mar 23, 2025
-
What Do Carbohydrates Do In The Cell Membrane
Mar 23, 2025
-
Applying Hardy Weinberg To Rock Pocket Mouse Field Data
Mar 23, 2025
-
Derivatives Of Logarithmic And Inverse Trigonometric Functions
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Net Ionic Equation For Acid Base Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
