Number Of Covalent Bonds In Carbon
Muz Play
Mar 20, 2025 · 7 min read
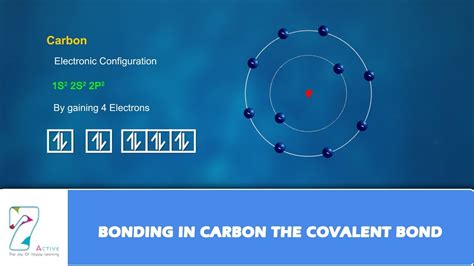
Table of Contents
The Curious Case of Carbon: Unveiling the Number of Covalent Bonds
Carbon, the cornerstone of organic chemistry and the building block of life itself, possesses a unique and fascinating bonding behavior. Unlike many other elements, carbon demonstrates a remarkable versatility in the number of covalent bonds it can form. This article delves deep into the intricacies of carbon's bonding, exploring the reasons behind its capacity to form multiple bonds and the profound implications this has on the diversity of organic molecules. We'll explore the factors governing bond formation, the different types of bonds carbon can create, and the impact this has on the structure and properties of countless compounds.
Understanding Covalent Bonding in Carbon
Carbon, with its electronic configuration of 1s²2s²2p², has four valence electrons in its outermost shell. These electrons are readily available for sharing with other atoms, leading to the formation of covalent bonds. A covalent bond arises from the mutual sharing of electron pairs between atoms. This sharing allows each atom to achieve a more stable electron configuration, typically resembling that of a noble gas (a full outer electron shell). For carbon, achieving this stability often means forming four covalent bonds.
The Octet Rule and Carbon's Bonding
The octet rule, a fundamental principle in chemistry, states that atoms tend to gain, lose, or share electrons in order to achieve a full set of eight valence electrons. Carbon, with its four valence electrons, needs to share four more electrons to satisfy the octet rule. This is why carbon readily forms four covalent bonds. This drive to fulfill the octet rule is the primary factor dictating carbon's bonding behavior.
Exceptions to the Octet Rule: Hypervalency and Hypovalency
While the octet rule provides a useful guideline, it's not without exceptions. In certain circumstances, carbon can exhibit hypervalency or hypovalency, deviating from the standard four bonds.
Hypervalency: Although rare for carbon, situations exist where it can seem to form more than four bonds. These are often best explained by considering resonance structures and delocalization of electrons. For example, certain carbocations (positively charged carbon ions) may appear to have more than four bonds when resonance structures are considered. However, in reality, the positive charge is delocalized, and no single carbon atom is genuinely bonded to more than four atoms.
Hypovalency: This refers to situations where carbon forms fewer than four bonds. Carbenes, for instance, are neutral molecules containing a divalent carbon atom (two bonds). These are highly reactive species due to their electron deficiency. Similarly, carbocations, with only three bonds to carbon, are also highly reactive intermediates in various organic reactions.
Types of Covalent Bonds Formed by Carbon
Carbon's ability to form multiple covalent bonds isn't limited to single bonds. Its versatility extends to double and triple bonds, significantly enriching the diversity of organic compounds.
Single Bonds (Sigma Bonds): The Foundation of Carbon's Structure
A single covalent bond, also known as a sigma (σ) bond, is formed by the direct head-on overlap of atomic orbitals. In carbon, this typically involves the overlap of one s orbital and three p orbitals. These sigma bonds are relatively strong and form the backbone of most organic molecules. Ethane (C₂H₆) provides a classic example of a molecule built entirely on single carbon-carbon and carbon-hydrogen sigma bonds.
Double Bonds (One Sigma, One Pi): Adding Unsaturation
A double bond consists of one sigma bond and one pi (π) bond. The sigma bond forms through head-on overlap, while the pi bond forms through the sideways overlap of p orbitals. This pi bond adds extra strength and rigidity to the double bond compared to a single bond. Ethylene (C₂H₄) is a prime example, showcasing the planar structure enforced by the carbon-carbon double bond. The presence of double bonds leads to "unsaturation" in organic molecules.
Triple Bonds (One Sigma, Two Pi): Maximum Bonding Capacity
A triple bond comprises one sigma bond and two pi bonds. Similar to double bonds, the sigma bond forms via head-on overlap, while the two pi bonds result from sideways overlap of two sets of p orbitals. Triple bonds are the strongest type of covalent bond carbon can form, imparting even greater rigidity and strength to the molecule. Acetylene (C₂H₂) exemplifies a molecule containing a carbon-carbon triple bond, characterized by its linear structure. The presence of triple bonds also signifies high unsaturation.
The Impact of Carbon's Bonding on Organic Chemistry
The ability of carbon to form four covalent bonds, including single, double, and triple bonds, has profound implications for the vastness and complexity of organic chemistry. This versatility allows carbon to form long chains, branched structures, and rings, leading to an almost limitless array of molecules with diverse properties.
Long Chains and Branched Structures: The Basis of Polymers
The capacity of carbon to form long chains via single bonds is the foundation for the existence of polymers, massive molecules comprising repeating structural units. These polymers are integral to many natural materials (e.g., proteins, DNA) and synthetic materials (e.g., plastics, rubbers). The ability to branch these chains further increases the diversity of possible structures and properties.
Rings and Cyclic Structures: A World of Possibilities
Carbon's propensity to form rings and cyclic structures significantly expands the landscape of organic molecules. Cyclic compounds exhibit distinct properties compared to their linear counterparts, influencing their reactivity and applications. Benzene, a classic example of an aromatic ring structure, plays a crucial role in many organic reactions and industrial processes.
Isomerism: Same Formula, Different Structure
Carbon's multifaceted bonding leads to the phenomenon of isomerism, where molecules share the same chemical formula but possess different structural arrangements. These isomers often exhibit vastly different physical and chemical properties, expanding the chemical diversity even further. Structural isomers, stereoisomers (geometric and optical), and conformational isomers are all a consequence of carbon's versatility in bonding.
Carbon's Bonding and the Origin of Life
Carbon's unique bonding capabilities are inextricably linked to the origin and evolution of life on Earth. The ability to form stable, complex molecules with diverse functionalities underpins the intricate structures and processes that characterize living organisms.
The Backbone of Biomolecules
Proteins, carbohydrates, lipids, and nucleic acids—the essential biomolecules of life—all rely on carbon's bonding capacity to form their complex structures. The long carbon chains, branched structures, and cyclic structures present in these biomolecules are crucial for their biological functions.
Carbon's Role in Energy Transfer
The ability of carbon to form stable bonds with oxygen and hydrogen allows for efficient energy storage and transfer in biological systems. Carbohydrates, for example, act as primary energy sources, storing energy in their carbon-carbon and carbon-oxygen bonds. The breakdown of these bonds releases energy for cellular processes.
The Carbon Cycle: A Continuous Exchange
The continuous cycling of carbon through the biosphere, atmosphere, and geosphere underscores the element's central role in maintaining life. Photosynthesis, respiration, and decomposition all involve the transformation of carbon-containing molecules, highlighting the element's dynamic role in the ecosystem.
Conclusion: Carbon's Enduring Significance
The number of covalent bonds carbon can form—up to four—is not merely a chemical detail but a fundamental characteristic that dictates the astonishing diversity of organic compounds and their paramount significance in the universe. The ability to form single, double, and triple bonds, along with the resultant capacity to construct long chains, branched structures, and rings, sets carbon apart and makes it the cornerstone of life and a vast range of materials with diverse applications. Further exploration into carbon's intricate bonding behavior will continue to illuminate new possibilities in materials science, medicine, and our understanding of the universe itself. The seemingly simple answer of "four" belies the extraordinary complexity and significance of carbon's bonding within the broader context of chemistry and biology.
Latest Posts
Latest Posts
-
The Mean Of The Sample Means
Mar 21, 2025
-
Is Sulfur More Electronegative Than Oxygen
Mar 21, 2025
-
Color The Neuron And Neuroglial Cells Answer Key
Mar 21, 2025
-
The Basic Unit Of Life Is The
Mar 21, 2025
-
Calorimetry And Hesss Law Pre Lab Answers
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Number Of Covalent Bonds In Carbon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
