Organic Compounds Contain Atoms Of What Element
Muz Play
Mar 30, 2025 · 5 min read
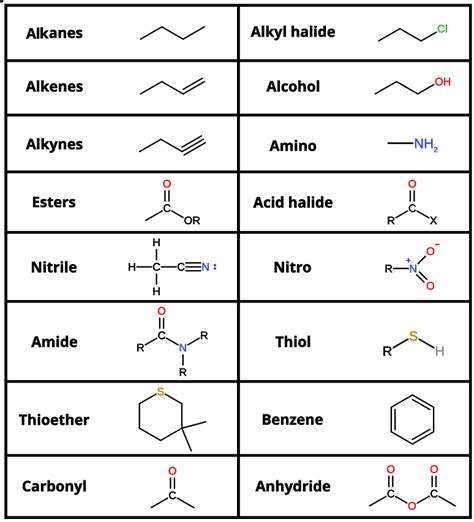
Table of Contents
Organic Compounds: A Deep Dive into the Carbon Atom's Central Role
Organic chemistry, a vast and fascinating field, is fundamentally defined by the presence of a specific element: carbon. While other elements play crucial supporting roles, carbon's unique properties make it the backbone of all organic compounds. This article delves into the reasons why carbon is so essential, exploring its bonding capabilities, the diversity of organic molecules it forms, and the implications for life on Earth and beyond.
The Uniqueness of Carbon: Why Carbon is King in Organic Chemistry
Carbon's dominance in organic chemistry stems from its exceptional ability to form strong covalent bonds with itself and other elements. This capacity arises from its electronic structure:
-
Four Valence Electrons: Carbon possesses four valence electrons, meaning it can form four covalent bonds. This tetravalency allows for the creation of a vast array of complex structures, including long chains, branched chains, and rings. No other element exhibits this versatility to the same extent.
-
Strong Covalent Bonds: Carbon forms strong, stable covalent bonds, leading to relatively stable molecules. These bonds are crucial for maintaining the structural integrity of biological molecules and other organic materials.
-
Catentation: Carbon atoms readily bond to other carbon atoms, a property known as catentation. This allows for the formation of long chains and complex ring structures, a foundation for the immense diversity seen in organic compounds. Silicon, another element with some capacity for catentation, falls far short of carbon's ability in this regard.
Comparing Carbon to other elements
To fully appreciate carbon's unique role, let's compare it briefly to other elements:
-
Silicon: Silicon, located directly beneath carbon in the periodic table, also has four valence electrons. However, the silicon-silicon bond is weaker than the carbon-carbon bond, limiting the size and complexity of silicon-based molecules. Consequently, silicon-based organic compounds, often called organosilicon compounds, are far less diverse than their carbon-based counterparts.
-
Other elements: While other elements can form covalent bonds, none possess the combination of strong covalent bonding ability, tetravalency, and catenation capacity that carbon does. This explains why the realm of organic chemistry is so overwhelmingly focused on carbon-containing compounds.
The Diversity of Organic Compounds: From Simple to Complex
The versatility of carbon leads to an astounding array of organic compounds, each with unique properties and functions. These molecules vary in size, shape, and functionality, ranging from simple hydrocarbons to complex biomolecules.
Hydrocarbons: The Building Blocks
Hydrocarbons, compounds containing only carbon and hydrogen, are fundamental building blocks of organic chemistry. They form the basis for many other organic compounds and serve as important fuels and raw materials.
-
Alkanes: These are saturated hydrocarbons, meaning they contain only single bonds between carbon atoms. Examples include methane (CH₄), ethane (C₂H₆), and propane (C₃H₈).
-
Alkenes: These unsaturated hydrocarbons contain at least one carbon-carbon double bond. Ethene (C₂H₄) is a common example.
-
Alkynes: These unsaturated hydrocarbons contain at least one carbon-carbon triple bond. Ethyne (C₂H₂) is a representative example.
-
Aromatic Hydrocarbons: These contain benzene rings, which are characterized by a delocalized electron system. Benzene (C₆H₆) is the simplest aromatic hydrocarbon.
Functional Groups: Adding Functionality
Functional groups are specific groups of atoms within a molecule that impart characteristic chemical properties. These groups significantly influence the reactivity and properties of organic compounds.
-
Alcohols (-OH): Alcohols contain a hydroxyl group (-OH) attached to a carbon atom. Ethanol (C₂H₅OH) is a common alcohol.
-
Carboxylic Acids (-COOH): Carboxylic acids contain a carboxyl group (-COOH). Acetic acid (CH₃COOH) is a well-known example.
-
Amines (-NH₂): Amines contain an amino group (-NH₂). Many biologically important molecules, such as amino acids, are amines.
-
Ketones (C=O): Ketones contain a carbonyl group (C=O) bonded to two carbon atoms. Acetone (CH₃COCH₃) is a common ketone.
-
Aldehydes (CHO): Aldehydes contain a carbonyl group (C=O) bonded to one carbon atom and one hydrogen atom. Formaldehyde (HCHO) is a simple aldehyde.
-
Esters (-COO-): Esters are derived from carboxylic acids and alcohols. Many esters have pleasant fragrances and are used in perfumes and flavorings.
The Role of Organic Compounds in Life
Organic chemistry is inextricably linked to life itself. All living organisms are composed primarily of organic compounds, which perform a myriad of essential functions.
Biomolecules: The Molecules of Life
Biomolecules are large organic molecules essential for life. These include:
-
Carbohydrates: These provide energy and structural support. Examples include glucose, starch, and cellulose.
-
Lipids: These are fats and oils, which store energy and form cell membranes.
-
Proteins: These are complex polymers of amino acids, performing diverse functions including catalysis, structural support, and transport.
-
Nucleic Acids: These carry genetic information (DNA and RNA).
The Importance of Isomerism
Isomers are molecules with the same molecular formula but different structures. This phenomenon is crucial in biology, as different isomers can have dramatically different properties and functions. For example, glucose and fructose have the same molecular formula (C₆H₁₂O₆) but different structures and properties.
Applications of Organic Chemistry: A Wide-Ranging Field
Organic chemistry isn't just confined to the study of life; it has far-reaching applications in many areas:
-
Medicine: The development of pharmaceuticals, including drugs to treat various diseases, heavily relies on organic chemistry. Understanding molecular structures and their interactions with biological systems is crucial for designing effective medications.
-
Materials Science: Organic compounds are used in the synthesis of polymers, plastics, fibers, and other materials with diverse applications.
-
Agriculture: Pesticides, herbicides, and fertilizers are often organic compounds designed to enhance crop yields and protect against pests.
-
Industry: Many industrial processes, such as the production of fuels, solvents, and dyes, rely on organic chemistry.
Conclusion: The Enduring Significance of Carbon in Organic Chemistry
In conclusion, the central role of carbon in organic chemistry is undeniable. Its unique properties of tetravalency, strong covalent bonding, and catenation allow for the formation of an incredibly diverse range of molecules. These molecules form the basis of life, drive many industrial processes, and continue to be the focus of ongoing research and discovery. From simple hydrocarbons to complex biomolecules, carbon's influence is pervasive and profound, making it the undisputed king of organic chemistry. The future of organic chemistry holds even more exciting advancements, as researchers continue to explore the potential of carbon-based compounds in addressing global challenges and developing innovative technologies. The study of organic chemistry is not just a study of molecules; it is a study of the very foundation of life and the materials that shape our world.
Latest Posts
Latest Posts
-
How To Find A Hamilton Circuit
Apr 01, 2025
-
The Positive Subatomic Particle Is The
Apr 01, 2025
-
Which Characteristic Of A Substance Is Considered A Chemical Property
Apr 01, 2025
-
What Major Change Occurs During Metamorphism Of Limestone To Marble
Apr 01, 2025
-
Do All Living Things Respond To Stimuli
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Organic Compounds Contain Atoms Of What Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
