Packing Efficiency Of Face Centered Cubic
Muz Play
Mar 31, 2025 · 6 min read
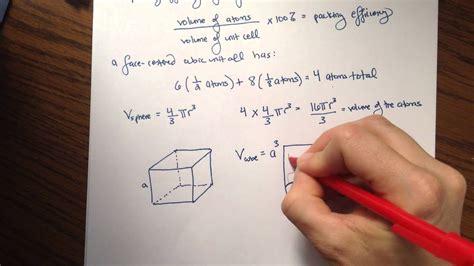
Table of Contents
Packing Efficiency of Face-Centered Cubic (FCC) Structures
The face-centered cubic (FCC) structure is a common crystal structure found in many metals and alloys. Understanding its packing efficiency is crucial in materials science, as it directly impacts material properties like density, ductility, and conductivity. This article delves deep into the intricacies of FCC packing, explaining its arrangement, calculating its efficiency, and comparing it to other crystal structures.
Understanding the FCC Structure
The FCC structure is characterized by atoms located at each corner of a cube and at the center of each face. Each corner atom is shared by eight adjacent unit cells, contributing 1/8 of an atom to each unit cell. Each face-centered atom is shared by two unit cells, contributing 1/2 of an atom to each unit cell. Therefore, a single FCC unit cell contains a total of 4 atoms:
- Corner atoms: 8 corners × (1/8 atom/corner) = 1 atom
- Face-centered atoms: 6 faces × (1/2 atom/face) = 3 atoms
- Total atoms per unit cell: 1 + 3 = 4 atoms
This arrangement leads to a highly efficient packing of atoms, maximizing the use of available space.
Visualization of the FCC Structure
Imagine stacking layers of spheres. In the first layer, spheres are arranged in a hexagonal close-packed (HCP) arrangement. The second layer sits in the depressions formed by the first layer. The crucial difference between FCC and HCP lies in the third layer. In FCC, the third layer's spheres are positioned directly above the spheres in the first layer, creating an ABCABC stacking sequence. This contrasts with HCP, which has an ABABAB stacking sequence.
Coordination Number and Atomic Radius
The coordination number in an FCC structure is 12. This means each atom is surrounded by 12 nearest neighbors. This high coordination number contributes to the structure's stability.
The relationship between the unit cell edge length (a) and the atomic radius (r) in an FCC structure is given by:
a = 2√2r
This equation is derived by considering the diagonal of a face of the unit cell, which passes through four atoms.
Calculating the Packing Efficiency of FCC
Packing efficiency is the percentage of volume within a unit cell that is occupied by atoms. For FCC, this calculation involves determining the volume occupied by atoms and the total volume of the unit cell.
Volume Occupied by Atoms
Since there are 4 atoms per unit cell, and assuming the atoms are perfect spheres, the total volume occupied by atoms is:
Volume occupied by atoms = 4 × (4/3)πr³
Total Volume of the Unit Cell
The total volume of the unit cell is given by the cube of the unit cell edge length:
Total volume = a³ = (2√2r)³ = 16√2r³
Packing Efficiency Calculation
The packing efficiency is then calculated as the ratio of the volume occupied by atoms to the total volume of the unit cell, multiplied by 100%:
Packing efficiency = [(Volume occupied by atoms) / (Total volume)] × 100%
Substituting the expressions for the volumes, we get:
Packing efficiency = [(4 × (4/3)πr³) / (16√2r³)] × 100%
After simplifying, the packing efficiency of an FCC structure is approximately:
Packing efficiency ≈ 74%
This means that approximately 74% of the volume in an FCC unit cell is occupied by atoms, leaving 26% as empty space.
Comparison with Other Crystal Structures
Let's compare the packing efficiency of FCC with other common crystal structures:
- Simple Cubic (SC): The simplest crystal structure, with atoms located only at the corners of the cube. Its packing efficiency is significantly lower at approximately 52%.
- Body-Centered Cubic (BCC): Atoms are located at the corners and the center of the cube. Its packing efficiency is around 68%.
- Hexagonal Close-Packed (HCP): Similar to FCC in its high density, HCP also exhibits a packing efficiency of approximately 74%. The difference lies in the stacking sequence of atomic layers.
This comparison highlights the high efficiency of FCC and HCP structures compared to SC and BCC.
Implications of Packing Efficiency
The high packing efficiency of the FCC structure has significant implications for the properties of materials that adopt this structure:
- Density: Higher packing efficiency translates to higher density. Materials with FCC structures tend to be denser than those with SC or BCC structures.
- Ductility: The close-packed nature of FCC allows for easier slip and deformation under stress, leading to greater ductility and malleability.
- Conductivity: The close proximity of atoms in FCC facilitates the movement of electrons, resulting in better electrical and thermal conductivity.
- Mechanical Strength: While ductility is high, the close-packed planes can also lead to certain weaknesses under specific stress conditions.
Advanced Concepts and Applications
The understanding of FCC packing extends beyond the basic calculation of efficiency. Several advanced concepts build upon this foundation:
Defects in FCC Structures
Real crystals are not perfect. Defects, such as vacancies, dislocations, and grain boundaries, affect material properties. The study of these defects in FCC structures is crucial for understanding material behavior under various conditions.
Alloying and FCC Structures
Many alloys exhibit FCC structures. Alloying elements can influence the stability and properties of the FCC structure, leading to tailored material properties for specific applications.
Applications of FCC Materials
Numerous materials with FCC structures find widespread applications:
- Aluminum: Used extensively in aerospace, automotive, and packaging industries due to its lightness and corrosion resistance.
- Copper: Used in electrical wiring, plumbing, and other applications requiring high electrical conductivity.
- Nickel: Used in alloys for high-temperature applications and corrosion resistance.
- Austenitic Stainless Steels: These alloys, with FCC structures, are known for their corrosion resistance and strength.
Influence of Temperature on FCC Structures
Some materials can undergo phase transformations between different crystal structures as temperature changes. The stability of the FCC structure can be influenced by temperature, affecting material properties at high or low temperatures.
Further Research and Developments
Ongoing research continues to refine our understanding of FCC structures, exploring their behavior under extreme conditions, and investigating novel applications of materials with FCC structures in emerging technologies. The development of advanced characterization techniques provides deeper insights into the atomic arrangements and defects within these structures, which helps in the development of improved materials with enhanced properties.
Conclusion
The face-centered cubic structure represents a highly efficient arrangement of atoms, maximizing space utilization and impacting various material properties. Its 74% packing efficiency, compared to other crystal structures, highlights its importance in materials science. Understanding the FCC structure and its implications is essential for developing and utilizing materials with tailored properties for diverse applications. The ongoing research and development in this area continue to expand our knowledge and lead to innovations in various fields. The detailed analysis presented here serves as a comprehensive introduction to the significance of FCC packing efficiency in the wider landscape of materials science and engineering.
Latest Posts
Latest Posts
-
The Process Of Independent Assortment Refers To
Apr 02, 2025
-
Is Table Salt Homogeneous Or Heterogeneous
Apr 02, 2025
-
What Part Of Bacteria Cell Helps It Move
Apr 02, 2025
-
Is The Organic Layer On The Top Or Bottom
Apr 02, 2025
-
In A Solution It Is Dissolving Medium
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Packing Efficiency Of Face Centered Cubic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
