Period 2 On The Periodic Table
Muz Play
Mar 15, 2025 · 7 min read
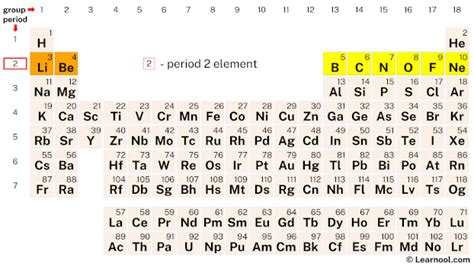
Table of Contents
Period 2: The Foundation of Chemical Diversity
Period 2 of the periodic table, encompassing the elements lithium (Li) to neon (Ne), holds a unique position in the world of chemistry. While seemingly small, this period represents a fundamental building block for understanding the trends and properties of elements across the entire table. Its elements showcase a remarkable range of properties, paving the way for the greater complexity observed in subsequent periods. This article will delve deep into Period 2, exploring the electronic configuration, chemical behavior, and unique characteristics of each element, highlighting its significance in various fields.
Electronic Configuration and the Significance of the 2nd Shell
The defining characteristic of Period 2 elements is their second electron shell. This shell, which can hold a maximum of eight electrons, dictates the chemical behavior and properties of these elements. Unlike Period 1, which only has the 1s orbital, Period 2 incorporates both the 2s and 2p orbitals. This addition significantly expands the potential for bonding and the variety of compounds these elements can form. The filling of these orbitals follows the Aufbau principle, with electrons first occupying the lower energy 2s orbital before moving to the higher energy 2p orbitals.
The Role of 2s and 2p Orbitals in Chemical Bonding
The 2s and 2p orbitals play a crucial role in the formation of chemical bonds. The 2s orbital is spherical and involved in sigma (σ) bonds, while the three 2p orbitals (2px, 2py, 2pz) are dumbbell-shaped and participate in both sigma (σ) and pi (π) bonds. The presence of p-orbitals enables the formation of multiple bonds, leading to a greater diversity of chemical structures and properties compared to Period 1 elements. This capacity for multiple bonding is critical for the formation of molecules crucial to life and various industrial processes.
Exploring Individual Elements of Period 2
Let's now explore each element in Period 2 individually, examining their properties and applications.
Lithium (Li): The Lightest Alkali Metal
Lithium, the first element in Period 2, is an alkali metal characterized by its low density, reactivity, and distinctive flame color (crimson red). Its single valence electron readily participates in chemical reactions, making it a powerful reducing agent. Lithium's unique properties have led to its widespread applications in various fields:
- Batteries: Lithium-ion batteries are ubiquitous in portable electronics, electric vehicles, and grid-scale energy storage due to Lithium's high energy density.
- Medicine: Lithium salts are used in the treatment of bipolar disorder.
- Ceramics and Glass: Lithium compounds are added to ceramics and glass to enhance their properties, such as improving thermal shock resistance.
Beryllium (Be): A Unique Alkaline Earth Metal
Beryllium, an alkaline earth metal, stands apart from its heavier congeners due to its small size and high ionization energy. Its exceptional strength-to-weight ratio and high melting point make it valuable in specialized applications:
- Aerospace: Beryllium alloys are used in aircraft and spacecraft components due to their lightness and strength.
- X-ray windows: Beryllium's transparency to X-rays makes it ideal for X-ray windows in medical and scientific instruments.
- Nuclear reactors: Beryllium is used as a neutron reflector in nuclear reactors.
Boron (B): A Metalloid with Diverse Applications
Boron, a metalloid, displays properties intermediate between metals and nonmetals. Its ability to form covalent bonds with a variety of elements leads to numerous applications:
- Semiconductors: Boron is a crucial dopant in semiconductors, influencing their electrical properties.
- Glass and ceramics: Boron compounds are essential components in the production of borosilicate glass (Pyrex), known for its heat resistance.
- Detergents and bleaches: Borax and boric acid find applications as detergents, bleaches, and in other cleaning products.
Carbon (C): The Foundation of Life
Carbon, a nonmetal, is arguably the most important element in Period 2 and indeed in the entire periodic table. Its ability to form four strong covalent bonds and to readily catenate (form chains and rings) allows it to create a vast array of organic molecules that are fundamental to all known life forms. Carbon's importance extends beyond biology:
- Fuels: Fossil fuels (coal, oil, and natural gas) are primarily composed of carbon-based compounds.
- Materials science: Carbon's allotropes (diamond and graphite) exhibit drastically different properties, highlighting its versatility. Graphene, a single layer of graphite, exhibits exceptional electrical conductivity and strength. Fullerenes and nanotubes represent new exciting frontiers in nanotechnology.
Nitrogen (N): An Essential Element for Life
Nitrogen, a nonmetal, is another crucial element for life. It makes up a significant portion of the Earth's atmosphere and is a vital component of amino acids, proteins, and nucleic acids (DNA and RNA). Industrially, nitrogen is vital for:
- Fertilizers: Ammonia (NH3), produced via the Haber-Bosch process, is the backbone of nitrogen fertilizers crucial for food production.
- Explosives: Many explosives, such as nitroglycerin and TNT, contain nitrogen.
- Refrigeration: Liquid nitrogen is used as a refrigerant in cryogenic applications.
Oxygen (O): Crucial for Respiration and Combustion
Oxygen, a nonmetal, is essential for respiration in nearly all living organisms. It is a highly reactive element that readily forms oxides with other elements. Its importance extends beyond biological processes:
- Combustion: Oxygen supports combustion, making it critical for various industrial processes and energy generation.
- Medicine: Oxygen therapy is widely used in medical settings to treat respiratory conditions.
- Water: Water (H2O) is a ubiquitous compound comprising two hydrogen atoms and one oxygen atom, crucial for life.
Fluorine (F): The Most Reactive Nonmetal
Fluorine, a nonmetal and the most electronegative element in the periodic table, is an extremely reactive element. Its high reactivity leads to applications in various fields, though safety precautions are paramount due to its corrosive nature.
- Dentistry: Fluoride compounds are commonly added to toothpaste and drinking water to prevent tooth decay.
- Refrigerants: Certain fluorocarbons were widely used as refrigerants before being phased out due to their ozone depletion potential.
- Industrial chemicals: Fluorine compounds are utilized in the production of various industrial chemicals and plastics.
Neon (Ne): A Noble Gas with Unique Properties
Neon, a noble gas, is a chemically inert element with limited reactivity. Its stable electronic configuration makes it unsuitable for chemical bonding under normal conditions. However, its unique properties make it valuable:
- Lighting: Neon's characteristic reddish-orange glow makes it widely used in neon signs.
- Lasers: Neon is used in various types of lasers.
- Cryogenics: Liquid neon is a refrigerant used in cryogenic applications.
Period 2 Trends and Their Significance
Period 2 exhibits clear trends in various properties, offering valuable insights into the behavior of elements:
- Atomic Radius: Atomic radius generally decreases across Period 2 from left to right due to increasing nuclear charge and limited shielding effect.
- Ionization Energy: Ionization energy generally increases across Period 2 from left to right due to the stronger attraction of the nucleus to the electrons.
- Electronegativity: Electronegativity, the tendency of an atom to attract electrons in a chemical bond, generally increases across Period 2 from left to right.
Understanding these trends is fundamental for predicting the chemical behavior and reactivity of the elements. This knowledge forms the basis for predicting the types of bonds elements will form, the stability of their compounds, and their overall chemical properties.
Conclusion: The Enduring Importance of Period 2
Period 2, despite encompassing only eight elements, represents a cornerstone of chemical understanding. Its elements demonstrate a remarkable range of properties, illustrating the fundamental principles that govern the behavior of all elements in the periodic table. From the lightweight lithium used in batteries to the life-sustaining carbon forming the basis of organic chemistry, and the inert neon lighting our cities, the elements of Period 2 have profoundly impacted our lives and continue to drive innovation across various scientific and technological fields. Further research and development in areas like material science and nanotechnology leveraging the unique properties of these elements promise even more exciting discoveries in the future. Understanding Period 2 is therefore not just a step in learning chemistry; it is the foundation upon which deeper understanding of the entire periodic table is built.
Latest Posts
Latest Posts
-
Adjusting Entries And Adjusted Trial Balance
May 09, 2025
-
What Is The Group Number For Sulfur
May 09, 2025
-
Which Stage Of Aerobic Respiration Requires An Input Of Oxygen
May 09, 2025
-
Five Carbon Sugar Found In Dna
May 09, 2025
-
The Oxygen Isotope With 8 Neutrons
May 09, 2025
Related Post
Thank you for visiting our website which covers about Period 2 On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.