Periodic Table With Gas Solid Liquid
Muz Play
Mar 22, 2025 · 6 min read
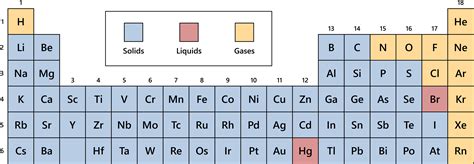
Table of Contents
The Periodic Table: A Deep Dive into Gases, Solids, and Liquids
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding this organization is crucial to comprehending the behavior of matter, particularly the differences between gases, solids, and liquids – the three fundamental states of matter. This comprehensive guide will explore the periodic table's structure, highlight the trends in physical states, and delve into the properties that determine whether an element exists as a gas, solid, or liquid at standard temperature and pressure (STP).
Understanding the Periodic Table's Structure
The periodic table's arrangement isn't arbitrary; it reflects the periodic recurrence of chemical properties. Elements are arranged in order of increasing atomic number (the number of protons in the nucleus). The table is divided into periods (horizontal rows) and groups (vertical columns). Elements within the same group share similar chemical properties due to similar valence electron configurations (the electrons in the outermost shell).
Periods and Trends:
Each period represents a principal energy level or shell. As you move across a period from left to right, the atomic number increases, and electrons are added to the same energy level. This leads to predictable trends in properties like electronegativity (the tendency to attract electrons in a bond) and ionization energy (the energy required to remove an electron).
Groups and Families:
Groups, also known as families, are columns of elements with similar chemical properties. These similarities stem from their identical number of valence electrons, leading to similar bonding behaviors. For example, Group 18 (noble gases) are notoriously unreactive because they have a full valence shell. Group 1 (alkali metals) are highly reactive because they readily lose one electron to achieve a stable electron configuration.
States of Matter: Gas, Solid, and Liquid
The state of matter of an element at STP is largely determined by the strength of intermolecular forces (forces between atoms or molecules) and the kinetic energy of its atoms or molecules.
1. Gases: Gases have weak intermolecular forces and high kinetic energy. Their atoms or molecules are widely dispersed and move freely and independently. This explains their ability to fill any container they occupy and their compressibility. Many elements exist as gases at STP, primarily those with low atomic numbers and weak interatomic forces.
2. Solids: Solids have strong intermolecular forces and low kinetic energy. Their atoms or molecules are tightly packed in a regular, ordered arrangement (crystalline structure). This arrangement accounts for their fixed shape and volume. Many metals, as well as some nonmetals, are solids at STP. The strength of the metallic bonds or covalent/ionic bonds determines the hardness and melting point of the solid.
3. Liquids: Liquids occupy an intermediate position between gases and solids. They have moderate intermolecular forces and moderate kinetic energy. Their atoms or molecules are close together but not rigidly fixed in position. This explains their ability to flow and assume the shape of their container, while maintaining a relatively constant volume. A few elements exist as liquids at STP, notably mercury (Hg) and bromine (Br).
Periodic Trends and States of Matter
The periodic table provides clues about an element's state at STP. Several trends are particularly relevant:
-
Atomic Size: As you move down a group, atomic size increases because electrons are added to higher energy levels further from the nucleus. Larger atoms generally lead to weaker intermolecular forces and a greater likelihood of a gaseous state.
-
Electronegativity: Electronegativity generally increases across a period and decreases down a group. Highly electronegative elements tend to form stronger intermolecular bonds, often resulting in solid states.
-
Melting and Boiling Points: These properties are directly related to the strength of intermolecular forces. Stronger forces lead to higher melting and boiling points. Elements with high melting and boiling points are usually solids at STP.
Examples of Elements in Different States at STP
Let's examine specific examples across the periodic table, categorizing elements based on their state at STP:
Gases at STP:
-
Group 18 (Noble Gases): Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and Radon (Rn) are all gases at STP. Their full valence shells result in extremely weak interatomic forces.
-
Hydrogen (H₂): A diatomic gas, hydrogen possesses weak covalent bonds between its atoms.
-
Nitrogen (N₂): Another diatomic gas with a triple bond, nitrogen is relatively unreactive due to the strength of this bond.
-
Oxygen (O₂): A diatomic gas vital for respiration. The double bond in oxygen is responsible for its gaseous nature.
-
Chlorine (Cl₂): A diatomic halogen gas, chlorine is highly reactive due to its tendency to gain an electron.
-
Fluorine (F₂): The most reactive halogen, fluorine, also exists as a diatomic gas.
Solids at STP:
-
Most Metals: The majority of metallic elements are solids at STP. The strong metallic bonding contributes to their solid states. Examples include iron (Fe), copper (Cu), gold (Au), and many others.
-
Many Nonmetals: Several nonmetals are solids at STP, including carbon (C), phosphorus (P), sulfur (S), and iodine (I). Covalent bonding networks, or strong van der Waals forces, hold these atoms together.
-
Metalloids: Metalloids exhibit properties of both metals and nonmetals. Silicon (Si) and germanium (Ge) are metalloids that exist as solids at STP.
Liquids at STP:
-
Mercury (Hg): The only metallic element liquid at STP. Its unique electronic configuration leads to weak metallic bonds, resulting in its liquid state.
-
Bromine (Br₂): A diatomic halogen, bromine is a reddish-brown liquid at STP. The relatively weak intermolecular forces between its molecules allow for a liquid state.
Exceptions and Considerations
While these trends provide a good general framework, exceptions exist. Factors like allotropy (different forms of an element, e.g., diamond and graphite for carbon) can significantly impact an element's state at STP. Pressure and temperature also play a crucial role. Changing these conditions can induce phase transitions between solid, liquid, and gas states.
Conclusion:
The periodic table is a powerful tool for understanding the relationships between elements and their properties, including their state of matter. While there are exceptions and considerations, the trends in atomic size, electronegativity, and intermolecular forces generally predict whether an element will exist as a gas, solid, or liquid at STP. This knowledge is essential for comprehending chemical reactions, material science, and various other fields. By understanding these fundamental relationships, we gain a deeper appreciation for the intricate organization and interconnectedness within the world of chemistry. Further exploration of the periodic table and its underlying principles will continue to unlock insights into the behavior and properties of matter. Remember that the state of an element is not absolute and can be altered by changes in pressure and temperature, adding another layer of complexity and fascination to the study of chemistry. Understanding these nuances is key to a complete appreciation of the periodic table and its role in explaining the physical world around us.
Latest Posts
Latest Posts
-
Yo Come Una Naranja Ayer Correct Incorrect
Mar 23, 2025
-
Recovery Time Sfter Heat Shock Bacterial Cell
Mar 23, 2025
-
Which Enzyme Catalyzes A Carbonyl Reduction
Mar 23, 2025
-
How Do You Increase The Potential Of A Capacitor
Mar 23, 2025
-
Responsibilities As A Citizen Of The United States
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Periodic Table With Gas Solid Liquid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
