Periodic Table With Metals Nonmetals And Metalloids
Muz Play
Mar 26, 2025 · 7 min read
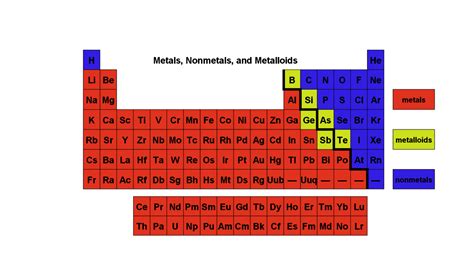
Table of Contents
Decoding the Periodic Table: A Deep Dive into Metals, Nonmetals, and Metalloids
The periodic table, a seemingly simple grid of elements, is a cornerstone of chemistry, revealing the fundamental building blocks of our universe and their intricate relationships. Understanding its structure, especially the categorization of elements into metals, nonmetals, and metalloids, is crucial for comprehending the vast spectrum of chemical properties and behaviors. This comprehensive guide delves into the intricacies of the periodic table, exploring the characteristics that define each category and highlighting their significance in various scientific fields.
The Organization of the Periodic Table: A Foundation for Understanding
The periodic table's genius lies in its organization. Elements are arranged in increasing order of atomic number (the number of protons in an atom's nucleus). This arrangement isn't arbitrary; it reflects recurring patterns in their chemical and physical properties, a phenomenon known as periodicity. Elements within the same vertical column, called a group or family, share similar properties due to their identical number of valence electrons (electrons in the outermost shell). Horizontal rows are called periods, and elements within the same period have the same number of electron shells.
Key Features and Regions of the Table
Several key features help navigate the periodic table:
- Metals: Occupying the vast majority of the table, metals are located to the left of the staircase-like line that separates them from nonmetals.
- Nonmetals: Situated to the right of the dividing line, these elements exhibit drastically different properties than metals.
- Metalloids (Semimetals): Positioned along the staircase line, these elements possess properties intermediate between metals and nonmetals.
- Alkali Metals (Group 1): Highly reactive metals with one valence electron.
- Alkaline Earth Metals (Group 2): Reactive metals with two valence electrons.
- Halogens (Group 17): Highly reactive nonmetals with seven valence electrons.
- Noble Gases (Group 18): Inert gases with full valence electron shells, making them exceptionally unreactive.
- Transition Metals: Located in the central block of the table, these metals exhibit a wide range of oxidation states and often form colorful compounds.
- Inner Transition Metals (Lanthanides and Actinides): Placed separately at the bottom of the table, these elements complete the filling of the f-sublevel.
Metals: The Characteristics of Conductivity and Malleability
Metals are characterized by a remarkable set of properties, stemming primarily from their electronic structure. Their valence electrons are loosely held, enabling them to easily move and conduct electricity and heat. This high electrical and thermal conductivity is a defining feature, making them indispensable in electrical wiring, heating elements, and numerous other applications.
Distinguishing Properties of Metals:
- Excellent Electrical Conductivity: Electrons flow freely through the metallic lattice, enabling the efficient transport of electrical charge.
- Excellent Thermal Conductivity: Heat energy is transferred rapidly throughout the metallic structure due to the mobile electrons.
- Malleability: Metals can be hammered or rolled into thin sheets without breaking, a testament to their ability to deform without fracturing.
- Ductility: Metals can be drawn into wires, demonstrating their capacity to be stretched without losing their structural integrity.
- Luster: Most metals possess a characteristic shiny appearance, reflecting light effectively.
- High Tensile Strength: Many metals exhibit significant resistance to breaking under tension.
- High Density: Metals generally have high densities compared to nonmetals.
- Sonorous: Many metals produce a ringing sound when struck.
Examples of Metals and Their Applications:
- Iron (Fe): A structural metal used in construction, machinery, and steel production. Its ability to form alloys with other elements significantly enhances its properties.
- Copper (Cu): An excellent conductor of electricity, extensively used in electrical wiring and plumbing.
- Aluminum (Al): Lightweight and corrosion-resistant, aluminum is crucial in aerospace, transportation, and packaging.
- Gold (Au) and Silver (Ag): Highly prized for their inertness, conductivity, and beauty, gold and silver are used in jewelry, electronics, and coinage.
- Titanium (Ti): Known for its high strength-to-weight ratio and corrosion resistance, titanium finds applications in aerospace, biomedical implants, and sporting goods.
Nonmetals: A Diverse Group with Varied Properties
In stark contrast to metals, nonmetals exhibit a diverse range of properties, often lacking the conductivity and malleability of their metallic counterparts. Their valence electrons are tightly bound to their atoms, resulting in significantly different chemical behaviors.
Defining Characteristics of Nonmetals:
- Poor Electrical Conductivity: Electrons are tightly bound, hindering the flow of electric current. Many are insulators.
- Poor Thermal Conductivity: Heat transfer is slow due to the limited electron mobility.
- Brittle: Nonmetals tend to shatter when subjected to stress, lacking the malleability of metals.
- Low Density: Generally less dense than metals.
- Dull Appearance: Lack the characteristic luster of metals.
- Various States at Room Temperature: Nonmetals can exist as solids, liquids (e.g., bromine), or gases (e.g., oxygen, nitrogen).
- High Ionization Energies: Requiring significant energy to remove an electron.
- High Electronegativity: A strong tendency to attract electrons in a chemical bond.
Examples of Nonmetals and Their Uses:
- Oxygen (O₂): Essential for respiration and combustion, oxygen is a vital component of the atmosphere.
- Nitrogen (N₂): A major constituent of the atmosphere, nitrogen is used in fertilizers and industrial processes.
- Carbon (C): The foundation of organic chemistry, carbon forms an immense variety of compounds, including diamonds, graphite, and numerous organic molecules.
- Chlorine (Cl₂): A highly reactive halogen used in water purification and the production of various chemicals.
- Sulfur (S): Used in the production of sulfuric acid, a crucial industrial chemical.
- Hydrogen (H₂): The lightest element, hydrogen is used as a fuel and in various chemical processes. It is also a key component of water.
- Phosphorus (P): Essential for biological systems, phosphorus is also used in fertilizers and matches.
Metalloids (Semimetals): Bridging the Gap Between Metals and Nonmetals
Metalloids occupy a fascinating middle ground, exhibiting properties intermediate between metals and nonmetals. Their behavior can be influenced by factors like temperature and pressure, leading to intriguing applications.
The Unique Characteristics of Metalloids:
- Semiconductors: Their electrical conductivity is intermediate, increasing with increasing temperature, a property exploited in semiconductor technology.
- Variable Properties: Their properties can vary depending on the specific conditions, making them adaptable to various applications.
- Brittle but less so than nonmetals: Possess some degree of malleability compared to nonmetals.
- Used in Semiconductors: Metalloids are used extensively in the electronics industry, as they can conduct electricity under specific conditions.
- Important in Alloys: Metalloids are added to alloys to improve their properties.
Examples of Metalloids and Their Significance:
- Silicon (Si): The backbone of the semiconductor industry, silicon is crucial for integrated circuits and microchips.
- Germanium (Ge): Used in transistors and other electronic components, particularly in high-frequency applications.
- Arsenic (As): Used in various alloys and as a doping agent in semiconductors.
- Boron (B): Used in high-strength materials, nuclear reactors, and semiconductors.
- Antimony (Sb): Used in alloys, such as lead-antimony alloys for batteries and type metal.
- Tellurium (Te): Used in solar cells and as a semiconductor.
The Periodic Table and its Impact on Various Scientific Fields
The periodic table isn't merely a classification system; it's a powerful tool with far-reaching implications across various scientific disciplines. Its predictive power allows scientists to anticipate the properties of newly discovered elements and design materials with specific characteristics.
Applications Across Disciplines:
- Materials Science: The table helps predict the properties of alloys and compounds, enabling the development of novel materials with desired properties.
- Chemistry: Understanding the trends in electronegativity, ionization energy, and reactivity allows chemists to predict reaction outcomes and design efficient synthetic routes.
- Physics: The table reveals patterns in electronic structure, enabling the study of atomic and molecular behavior.
- Biochemistry: The table highlights the essential elements for biological systems, allowing biologists to understand the roles of various elements in living organisms.
- Environmental Science: The table helps identify toxic elements and understand their environmental impact.
- Nuclear Chemistry: The table is vital for understanding nuclear reactions and the properties of radioactive isotopes.
Conclusion: A Continuing Journey of Discovery
The periodic table, with its intricate organization and wealth of information, remains a vital tool for scientists and students alike. Understanding the fundamental differences and similarities between metals, nonmetals, and metalloids provides a crucial foundation for comprehending the vast landscape of chemical and physical properties. As our knowledge of the elements expands, the periodic table will continue to evolve, revealing deeper insights into the building blocks of our universe and their intricate interrelationships. Further exploration into the periodic trends, chemical bonding, and reactivity patterns further solidifies the table's indispensable role in scientific discovery and technological advancement.
Latest Posts
Latest Posts
-
Essentials Of Oceanography Pdf Free Download
Mar 29, 2025
-
Lyrics Of Ode To Billy Joe
Mar 29, 2025
-
What Is The Distance Between And On The Number Line
Mar 29, 2025
-
Limit Of A Function Of Two Variables
Mar 29, 2025
-
The Short Run Phillips Curve Implies There Is A Trade Off Between
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Periodic Table With Metals Nonmetals And Metalloids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
