Protein Separation By Ion Exchange Chromatography
Muz Play
Apr 06, 2025 · 5 min read
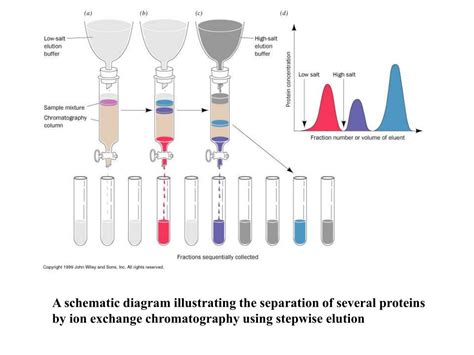
Table of Contents
Protein Separation by Ion Exchange Chromatography: A Comprehensive Guide
Ion exchange chromatography (IEC) is a powerful and versatile technique widely used for separating and purifying proteins based on their net surface charge. This method leverages the electrostatic interactions between charged protein molecules and oppositely charged ion exchange resins packed within a chromatography column. This comprehensive guide delves into the principles, techniques, and applications of protein separation using IEC.
Understanding the Fundamentals of Ion Exchange Chromatography
At the heart of IEC lies the principle of electrostatic attraction. Proteins, being complex macromolecules, possess a variety of charged amino acid residues on their surface. The net charge of a protein depends on its amino acid composition and the pH of the surrounding solution. This net charge is crucial for separation via IEC.
The chromatography column is packed with a stationary phase, a resin consisting of a porous matrix (often agarose or cellulose) to which charged functional groups are covalently attached. These functional groups can be either anionic (negatively charged) or cationic (positively charged), determining the type of IEC employed:
-
Anion Exchange Chromatography (AEC): Uses a stationary phase with positively charged functional groups (e.g., diethylaminoethyl, DEAE). Proteins with net negative charges (at a given pH) will bind to the resin, while positively charged proteins will flow through the column.
-
Cation Exchange Chromatography (CEC): Employs a stationary phase with negatively charged functional groups (e.g., carboxymethyl, CM). Proteins with net positive charges (at a given pH) will bind to the resin, while negatively charged proteins will elute in the void volume.
Key Factors Influencing Protein Separation by IEC
Several factors significantly impact the efficiency and selectivity of protein separation by IEC:
1. pH of the Mobile Phase (Buffer):
The pH of the buffer solution plays a crucial role in determining the net charge of the proteins. Adjusting the pH allows for precise control over the binding and elution of proteins. At a pH above the protein's isoelectric point (pI), the protein will carry a net negative charge and bind to an AEC column. Conversely, below the pI, it will carry a net positive charge and bind to a CEC column.
2. Ionic Strength of the Mobile Phase:
The ionic strength of the buffer, often controlled by adding salts like NaCl or KCl, affects the competition for binding sites on the resin. A high ionic strength can disrupt the electrostatic interactions between the protein and the resin, leading to elution. A gradient of increasing ionic strength is commonly used to elute bound proteins with increasing levels of charge density.
3. Choice of Resin:
Various ion exchange resins are available, differing in their matrix material, functional group density, and pore size. The choice of resin significantly influences the resolution and capacity of the separation. Factors to consider include:
-
Matrix Material: Agarose, cellulose, and polystyrene are common matrix materials, each exhibiting different properties regarding flow rates, pressure tolerance, and binding capacity.
-
Functional Group Density: Higher density means greater binding capacity but potentially lower resolution.
-
Pore Size: Appropriate pore size is essential to allow access of the proteins to the binding sites.
4. Temperature:
Temperature can influence protein stability and binding affinity. While usually not a primary factor for optimization, temperature control can be crucial for separating labile proteins.
Step-by-Step Procedure for Protein Separation by Ion Exchange Chromatography
The process typically involves several key steps:
1. Sample Preparation: The protein sample should be appropriately buffered to match the starting conditions of the chosen chromatography column. Centrifugation or filtration may be necessary to remove particulate matter.
2. Column Equilibration: The ion exchange column is equilibrated with the starting buffer to ensure optimal conditions for protein binding.
3. Sample Loading: The prepared protein sample is carefully loaded onto the equilibrated column.
4. Washing: After sample loading, a wash step with the starting buffer removes unbound proteins.
5. Elution: Bound proteins are eluted using a gradient of increasing ionic strength, pH, or a combination of both. This step separates the proteins based on their binding affinities. Fractions are collected during this process.
6. Analysis: The collected fractions are analyzed to determine the presence and purity of the target protein. Techniques like SDS-PAGE, Western blotting, or spectrophotometry are commonly employed.
7. Pooling and Concentration: Fractions containing the purified target protein are pooled together, and the protein concentration is increased using techniques such as ultrafiltration.
Advanced Techniques and Considerations
Several advanced techniques enhance the power and efficiency of IEC:
-
Gradient Elution: Instead of a step-wise increase in ionic strength, a gradual gradient is more effective in separating proteins with similar charge properties.
-
High-Performance Ion Exchange Chromatography (HP-IEC): This technique uses smaller particle resins and higher pressures, leading to faster separations and improved resolution.
-
Multidimensional Chromatography: Combining IEC with other chromatographic techniques, such as size exclusion or affinity chromatography, can improve the purity and yield of protein separation.
-
Immobilized Metal Affinity Chromatography (IMAC): This technique often precedes or follows IEC for increased selectivity. IMAC uses metal ions (like Nickel or Cobalt) to capture proteins with Histidine tags.
Applications of Ion Exchange Chromatography in Protein Purification
IEC finds widespread application across diverse areas of protein research and biotechnology:
-
Pharmaceutical Industry: Purification of therapeutic proteins (e.g., antibodies, enzymes, hormones) from complex biological sources.
-
Biotechnology: Purification of recombinant proteins produced using microbial or mammalian cell expression systems.
-
Proteomics: Separating and identifying proteins from complex biological samples for comprehensive protein analysis.
-
Food Science: Purification and characterization of food proteins.
-
Environmental Science: Separation and analysis of proteins from environmental samples for detecting pollutants.
-
Clinical Diagnostics: Purification of proteins from body fluids for diagnostic purposes.
Troubleshooting Common Issues in Ion Exchange Chromatography
Despite its robustness, some challenges can arise during IEC:
-
Low Protein Recovery: Insufficient equilibration, inappropriate buffer conditions, or protein degradation may cause low recovery.
-
Poor Resolution: Inadequate gradient design, overloaded columns, or inappropriate resin choice can lead to poor resolution.
-
Protein Aggregation: High protein concentrations or harsh elution conditions may lead to protein aggregation.
Conclusion
Ion exchange chromatography is a cornerstone technique for the separation and purification of proteins. Its versatility, high resolution, and scalability make it invaluable in numerous applications. By carefully controlling parameters like pH, ionic strength, and resin selection, researchers can achieve highly efficient purification of target proteins, contributing significantly to advancements in biotechnology, pharmaceuticals, and other related fields. Understanding the underlying principles and adapting the techniques appropriately is crucial for successful protein purification using IEC. Careful optimization and attention to detail are key to maximizing the effectiveness of this powerful separation method.
Latest Posts
Latest Posts
-
Plants Are Photosynthetic Autotrophs What Does This Mean
Apr 07, 2025
-
Equilibrium And Le Chateliers Principle Lab Answer Key
Apr 07, 2025
-
Bile Is Stored And Concentrated In The
Apr 07, 2025
-
Moment Of Inertia Rod About Center
Apr 07, 2025
-
Was Adolf Hitler A Great Leader
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Protein Separation By Ion Exchange Chromatography . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
