Provide The Formula For Each Compound.
Muz Play
Mar 15, 2025 · 6 min read
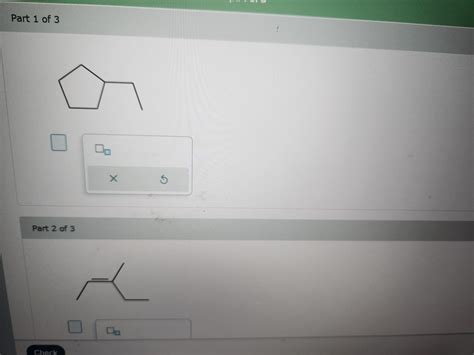
Table of Contents
The Amazing World of Chemical Formulas: A Comprehensive Guide
Understanding chemical formulas is fundamental to grasping the world of chemistry. A chemical formula is a concise way of representing the atoms present in a chemical compound, showing the types and numbers of atoms involved. This article delves deep into the fascinating realm of chemical formulas, providing a detailed explanation of how they're written, what they represent, and examples illustrating their use. We'll cover various types of formulas, including empirical formulas, molecular formulas, and structural formulas, providing the formula for a wide array of compounds.
Understanding the Basics: Elements and Symbols
Before diving into the formulas themselves, it's crucial to understand the foundation: the elements and their symbols. Elements are pure substances that cannot be broken down into simpler substances by chemical means. Each element is represented by a unique symbol, usually one or two letters derived from its name (e.g., H for hydrogen, O for oxygen, C for carbon, Na for sodium). These symbols are the building blocks of chemical formulas.
Empirical Formulas: The Simplest Representation
The empirical formula of a compound provides the simplest whole-number ratio of atoms present in the compound. It doesn't necessarily reflect the actual number of atoms in a molecule, but rather the ratio between them. For example, the empirical formula of hydrogen peroxide (H₂O₂) is HO, indicating a 1:1 ratio of hydrogen to oxygen atoms. This is because the simplest whole-number ratio of 2:2 can be reduced to 1:1.
Examples of Empirical Formulas:
- Water (H₂O): The empirical formula is also H₂O. The ratio of hydrogen to oxygen is 2:1.
- Glucose (C₆H₁₂O₆): The empirical formula is CH₂O. The ratio of carbon, hydrogen, and oxygen is 1:2:1.
- Benzene (C₆H₆): The empirical formula is CH. The ratio of carbon to hydrogen is 1:1.
Molecular Formulas: Revealing the Actual Composition
The molecular formula shows the actual number of each type of atom present in a molecule. It's a more detailed representation than the empirical formula. For glucose, the molecular formula is C₆H₁₂O₆, revealing that each molecule contains six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. In cases where the empirical and molecular formulas are the same (like water), it simply means that the simplest ratio also represents the actual composition of the molecule.
Examples of Molecular Formulas:
- Water (H₂O): The molecular formula is H₂O.
- Carbon Dioxide (CO₂): The molecular formula is CO₂.
- Methane (CH₄): The molecular formula is CH₄.
- Ethane (C₂H₆): The molecular formula is C₂H₆.
- Ethanol (C₂H₅OH): The molecular formula is C₂H₆O.
Structural Formulas: Visualizing the Arrangement
Structural formulas go beyond simply indicating the types and numbers of atoms; they also depict how the atoms are arranged within the molecule. They show the bonds between atoms, providing valuable information about the molecule's shape and properties. This is particularly important for organic compounds, which often exhibit isomerism (compounds with the same molecular formula but different structural arrangements).
Examples of Structural Formulas:
- Methane (CH₄): A central carbon atom bonded to four hydrogen atoms in a tetrahedral arrangement.
- Ethane (C₂H₆): Two carbon atoms bonded together, each bonded to three hydrogen atoms.
- Ethanol (C₂H₅OH): Two carbon atoms bonded together, one bonded to an -OH group (hydroxyl group) and the other bonded to three hydrogen atoms.
Ionic Compounds: Formulas for Charged Species
Ionic compounds are formed by the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions). Their formulas reflect the ratio of cations to anions needed to achieve electrical neutrality. The formula is written by indicating the cation first, followed by the anion. Subscripts are used to balance the charges.
Examples of Ionic Compound Formulas:
- Sodium Chloride (NaCl): Sodium (Na⁺) and chloride (Cl⁻) ions combine in a 1:1 ratio.
- Magnesium Oxide (MgO): Magnesium (Mg²⁺) and oxide (O²⁻) ions combine in a 1:1 ratio.
- Aluminum Oxide (Al₂O₃): Aluminum (Al³⁺) and oxide (O²⁻) ions combine in a 2:3 ratio.
- Calcium Chloride (CaCl₂): Calcium (Ca²⁺) and chloride (Cl⁻) ions combine in a 1:2 ratio.
- Ammonium Nitrate (NH₄NO₃): Ammonium (NH₄⁺) and nitrate (NO₃⁻) ions combine in a 1:1 ratio.
Polyatomic Ions: Handling Groups of Atoms
Polyatomic ions are groups of atoms that carry an overall charge. They are treated as single units when writing chemical formulas. The charges of polyatomic ions are crucial in determining the correct subscripts in the formula.
Examples of Compounds with Polyatomic Ions:
- Sodium Sulfate (Na₂SO₄): Sodium (Na⁺) and sulfate (SO₄²⁻) ions combine in a 2:1 ratio.
- Potassium Phosphate (K₃PO₄): Potassium (K⁺) and phosphate (PO₄³⁻) ions combine in a 3:1 ratio.
- Calcium Carbonate (CaCO₃): Calcium (Ca²⁺) and carbonate (CO₃²⁻) ions combine in a 1:1 ratio.
- Ammonium Sulfate ((NH₄)₂SO₄): Ammonium (NH₄⁺) and sulfate (SO₄²⁻) ions combine in a 2:1 ratio.
Hydrates: Incorporating Water Molecules
Hydrates are compounds that contain water molecules within their crystal structure. The number of water molecules associated with each formula unit is indicated using a dot (·) followed by the number of water molecules.
Examples of Hydrate Formulas:
- Copper(II) sulfate pentahydrate (CuSO₄·5H₂O): Each formula unit of copper(II) sulfate contains five water molecules.
- Epsom salt (MgSO₄·7H₂O): Each formula unit of magnesium sulfate contains seven water molecules.
Predicting Formulas: Using Oxidation States
Understanding oxidation states (or oxidation numbers) helps predict the formulas of ionic compounds. The oxidation states of the elements involved determine the ratio of ions needed to balance the charges. The sum of oxidation states in a neutral compound must be zero.
Beyond the Basics: Advanced Formula Representation
For complex molecules, various advanced representations exist, including:
- Condensed Structural Formulas: These formulas show the arrangement of atoms in a more compact form than full structural formulas, often omitting explicit bonds but showing the connectivity of atoms. For example, ethanol can be represented as CH₃CH₂OH.
- Skeletal Formulas (Line-angle Formulas): These are simplified representations used primarily in organic chemistry where carbon atoms are implied at the intersections and ends of lines, and hydrogen atoms attached to carbons are omitted.
- 3D Representations: These visually represent the three-dimensional structure of molecules, including bond angles and spatial orientations. Various methods exist for this, such as ball-and-stick models and space-filling models.
Conclusion: Mastering the Language of Chemistry
Chemical formulas are the cornerstone of chemical communication. Mastering their various forms – from simple empirical formulas to complex structural representations – is essential for understanding and working with chemical compounds. This comprehensive guide provides a strong foundation for further exploration into the fascinating world of chemistry. Remember that practice is key; work through examples and try writing formulas for different compounds to solidify your understanding. By understanding these principles, you can decipher the language of chemistry and gain valuable insights into the composition and structure of matter.
Latest Posts
Latest Posts
-
What Is The N Type Semiconductor
May 09, 2025
-
How Do Objects Become Negatively Charged
May 09, 2025
-
Binary Molecular Compounds Are Made Of Two
May 09, 2025
-
Which Is Present Only In Eukaryotic Cells
May 09, 2025
-
What Domain Is Kingdom Animalia In
May 09, 2025
Related Post
Thank you for visiting our website which covers about Provide The Formula For Each Compound. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.