Pxidation Number Of H In H20
Muz Play
Mar 17, 2025 · 5 min read
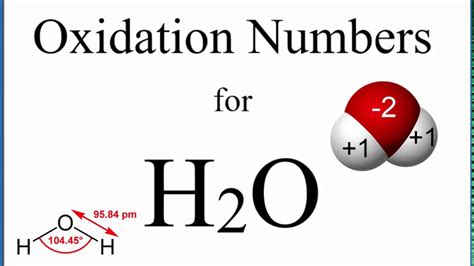
Table of Contents
Understanding the Oxidation Number of Hydrogen in H₂O
The seemingly simple molecule of water, H₂O, offers a fascinating glimpse into the complexities of oxidation numbers. While often taken for granted, understanding the oxidation number of hydrogen in H₂O is crucial for comprehending various chemical reactions and concepts, from redox reactions to the behavior of acids and bases. This comprehensive guide will delve into the definition of oxidation numbers, the rules for assigning them, and specifically focus on the oxidation state of hydrogen in water and its implications.
What is an Oxidation Number?
An oxidation number, also known as an oxidation state, represents the hypothetical charge an atom would have if all bonds to atoms of different elements were 100% ionic. It's a crucial tool in chemistry used to track electron transfers in chemical reactions, particularly redox reactions (reduction-oxidation reactions). It's important to note that oxidation numbers are assigned using a set of rules, and they are not necessarily the actual charges on atoms in a molecule. They are a bookkeeping system that simplifies the analysis of complex electron distributions.
Rules for Assigning Oxidation Numbers
Assigning oxidation numbers follows a specific set of rules, prioritized in the order listed below:
-
Free elements: The oxidation number of an atom in its elemental form is always 0. For example, the oxidation number of O in O₂ is 0, and the oxidation number of H in H₂ is 0.
-
Monatomic ions: The oxidation number of a monatomic ion is equal to its charge. For example, the oxidation number of Na⁺ is +1, and the oxidation number of Cl⁻ is -1.
-
Fluorine: Fluorine, the most electronegative element, always has an oxidation number of -1 in its compounds.
-
Oxygen: Oxygen usually has an oxidation number of -2 in its compounds. Exceptions include peroxides (e.g., H₂O₂, where oxygen has an oxidation number of -1) and compounds with oxygen bonded to fluorine (e.g., OF₂, where oxygen has a positive oxidation number).
-
Hydrogen: Hydrogen usually has an oxidation number of +1 in its compounds. An exception is when it is bonded to metals (e.g., in metal hydrides like NaH), where it has an oxidation number of -1.
-
The sum of oxidation numbers: In a neutral molecule, the sum of the oxidation numbers of all atoms is 0. In a polyatomic ion, the sum of the oxidation numbers equals the charge of the ion.
Determining the Oxidation Number of Hydrogen in H₂O
Applying these rules to H₂O, we can determine the oxidation number of hydrogen:
- Oxygen: Oxygen typically has an oxidation number of -2 (rule 4).
- Water is a neutral molecule: The sum of oxidation numbers must be 0 (rule 6).
- Let 'x' represent the oxidation number of hydrogen: Since there are two hydrogen atoms, the total contribution from hydrogen is 2x.
Therefore, we can set up the equation:
2x + (-2) = 0
Solving for x:
2x = 2 x = +1
Thus, the oxidation number of hydrogen in H₂O is +1.
Implications of Hydrogen's Oxidation Number in H₂O
The +1 oxidation state of hydrogen in water has several significant implications:
1. Redox Reactions
Understanding the oxidation number of hydrogen is fundamental to analyzing redox reactions involving water. In reactions where water acts as an oxidizing agent, hydrogen's oxidation number changes from +1 to 0 (as in the reduction of water to hydrogen gas). Conversely, when water acts as a reducing agent, the oxygen undergoes a change in its oxidation number, while hydrogen remains at +1.
2. Acid-Base Chemistry
The +1 oxidation state of hydrogen plays a key role in defining acids and bases according to the Brønsted-Lowry theory. Acids are proton (H⁺) donors, and the hydrogen ion carries a +1 charge, reflecting its oxidation state in many compounds, including water.
3. Electrochemical Reactions
Electrochemical reactions, such as those occurring in fuel cells, often involve water. The oxidation number of hydrogen in water helps predict the potential of half-cell reactions and the overall cell potential.
4. Understanding Chemical Bonding
While oxidation numbers are a hypothetical charge, they provide insights into the relative electronegativity of atoms within a molecule. The +1 oxidation number of hydrogen in H₂O reflects the higher electronegativity of oxygen, which attracts the shared electrons more strongly, leading to a partial positive charge on hydrogen. This understanding helps predict the polarity of the water molecule and its unique properties.
Exceptions and Ambiguities in Oxidation Numbers
It's essential to acknowledge that while the rules for assigning oxidation numbers are generally straightforward, there can be ambiguities and exceptions, particularly in complex molecules or those with unusual bonding arrangements. In such cases, the assigned oxidation numbers may not perfectly reflect the actual charge distribution within the molecule. However, even with these limitations, oxidation numbers remain a powerful tool for understanding and predicting chemical behavior.
Advanced Concepts and Applications
The concept of oxidation numbers extends beyond the simple case of H₂O. More advanced applications involve:
-
Complex coordination compounds: Assigning oxidation numbers in coordination complexes, where a central metal ion is surrounded by ligands, requires careful consideration of the charges and oxidation states of the ligands.
-
Organic chemistry: Oxidation numbers are used extensively in organic chemistry to track electron changes during oxidation and reduction reactions, including those involving functional groups like alcohols, aldehydes, and ketones.
-
Inorganic chemistry: Oxidation numbers are fundamental to understanding the behavior of transition metals and their diverse oxidation states, which are responsible for the rich chemistry of these elements.
-
Redox titrations: The accurate determination of oxidation numbers is critical for performing redox titrations, a quantitative analytical technique used to determine the concentration of unknown solutions.
Conclusion
The oxidation number of hydrogen in H₂O, a seemingly simple molecule, holds significant implications across various branches of chemistry. Understanding this seemingly small detail allows for a deeper comprehension of chemical bonding, redox reactions, acid-base chemistry, and electrochemical processes. While there are exceptions and complexities, mastering the rules for assigning oxidation numbers is an essential skill for any chemist, whether a student or a seasoned professional. The concepts explored in this article lay a strong foundation for more advanced studies in chemistry and further enhance your understanding of the fundamental principles governing the world around us. By consistently applying these rules and understanding their limitations, you'll gain a powerful analytical tool for interpreting and predicting the behavior of countless chemical species.
Latest Posts
Latest Posts
-
Which Body Cavity Protects The Spinal Column
Mar 17, 2025
-
Sampling Distribution Of The Sample Mean Calculator
Mar 17, 2025
-
Expected Value Of A Discrete Random Variable
Mar 17, 2025
-
Calculate Generation Time From Growth Curve
Mar 17, 2025
-
Factors That Affect Elasticity Of Supply
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Pxidation Number Of H In H20 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
