Question Lexan Draw The Monomer Used To Make This Polymer
Muz Play
Mar 26, 2025 · 6 min read
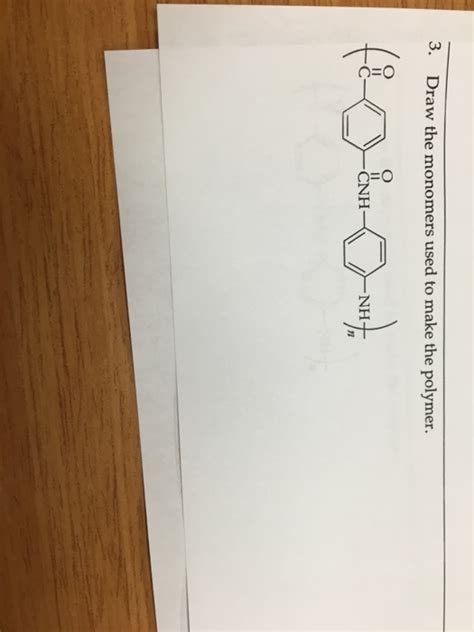
Table of Contents
Lexan: Delving into the Monomer and Polymerization Process
Lexan, a polycarbonate renowned for its exceptional strength, impact resistance, and optical clarity, finds widespread applications in various industries. From safety eyewear and automotive components to medical devices and consumer electronics, its unique properties make it an indispensable material. Understanding the fundamental building blocks of Lexan – its monomer – is key to appreciating its remarkable characteristics. This article will comprehensively explore the monomer used to produce Lexan, the polymerization process, and the resulting polymer's properties.
The Monomer: Bisphenol A (BPA)
The primary monomer used in the synthesis of Lexan is bisphenol A (BPA). Chemically, BPA is a diphenol compound, meaning it possesses two hydroxyl (-OH) groups. Its full chemical name is 2,2-bis(4-hydroxyphenyl)propane. The structure of BPA is crucial to understanding the polymerization process.
Understanding the BPA Structure
The BPA molecule consists of two phenol rings connected by a propane bridge (isopropyl group). The presence of these hydroxyl groups is paramount, as they are the reactive sites that participate in the polymerization reaction. This specific structure is responsible for the rigid, yet impact-resistant, properties of the resulting polycarbonate.
Chemical Formula: C<sub>15</sub>H<sub>16</sub>O<sub>2</sub>
Structural Formula:
OH OH
| |
-C-C-C- -C-C-C-
| |
Benzene Ring Benzene Ring
BPA's Role in Polymerization
The two hydroxyl groups in BPA are essential for the formation of ester linkages during the polymerization process. These linkages create the long chains that characterize the polycarbonate structure. The reactivity of these hydroxyl groups, combined with the steric hindrance provided by the isopropyl bridge, influences the kinetics and the final properties of the polymer.
The Polymerization Process: Transesterification
The synthesis of Lexan involves a transesterification reaction. This is a type of esterification reaction where an ester reacts with an alcohol to produce a new ester and a new alcohol. In the case of Lexan production, the reaction involves BPA and diphenyl carbonate (DPC). This reaction is typically carried out in the presence of a catalyst, often a metallic catalyst such as a titanium or zinc compound.
Step-by-Step Breakdown
-
Initiation: The reaction begins with the interaction between the hydroxyl groups of BPA and the carbonyl groups of DPC. The catalyst facilitates the cleavage of the ester bond in DPC.
-
Chain Growth: This initial reaction forms a new ester linkage between BPA and DPC, creating a dimer (a molecule formed from two monomers). The newly formed dimer still possesses reactive hydroxyl groups at its ends, allowing it to continue reacting with additional DPC molecules. This process continues, adding more BPA units to the growing chain.
-
Chain Termination: The chain growth continues until the reaction is terminated. This can be achieved by carefully controlling the reaction conditions, such as temperature and the concentration of the reactants. The termination step results in the formation of a long chain polycarbonate molecule with distinct end groups.
The Role of the Catalyst
The catalyst is crucial for efficient transesterification. It accelerates the reaction rate by lowering the activation energy required for the bond breakage and formation. The choice of catalyst influences both the reaction rate and the polymer's properties. Different catalysts can lead to different molecular weight distributions and thus affect the mechanical and thermal properties of the resulting Lexan.
Properties of Lexan Polycarbonate
The unique properties of Lexan are a direct consequence of its chemical structure and the polymerization process. Let's explore some of its key characteristics:
High Impact Resistance
The strong covalent bonds within the polycarbonate backbone, along with the flexibility imparted by the isopropyl bridge in the BPA monomer, give Lexan its superior impact resistance. This makes it ideal for applications requiring durability and resistance to shock.
High Tensile Strength
The strong carbon-oxygen-carbon bonds in the ester linkages contribute to Lexan's exceptional tensile strength. This means it can withstand significant stress before breaking, making it suitable for structural applications.
Excellent Optical Clarity
The regular and symmetrical arrangement of the polymer chains in Lexan allows for high light transmission, resulting in its optical clarity. This property is exploited in applications like safety eyewear and lenses.
Good Thermal Stability
Lexan exhibits excellent thermal stability, capable of withstanding elevated temperatures without significant degradation. This is essential for applications in harsh environments or those exposed to heat.
Dimensional Stability
Lexan demonstrates good dimensional stability, meaning it maintains its shape and size even under varying temperature and humidity conditions. This is important for applications requiring precise dimensions.
Chemical Resistance
Lexan demonstrates good resistance to a variety of chemicals, although it's not universally resistant. Its resistance depends on the specific chemical and its concentration. The stability of the carbonate linkages contributes to this resistance.
Applications of Lexan Polycarbonate
Lexan's unique combination of properties has led to its wide use in diverse industries:
Safety Equipment
Lexan's impact resistance makes it ideal for safety glasses, shields, and other protective equipment, ensuring the protection of individuals against hazardous impacts.
Automotive Industry
In the automotive industry, Lexan is used in various components including headlamp lenses, instrument panels, and exterior body parts due to its light weight and high durability.
Medical Devices
Its biocompatibility and sterilizability make it suitable for medical devices such as surgical instruments, catheter housings, and other components requiring both strength and biocompatibility.
Consumer Electronics
Lexan finds application in consumer electronics components such as mobile phone housings, laptop casings, and television screens due to its strength, light weight, and scratch resistance.
Other Applications
Beyond the aforementioned industries, Lexan is used in construction, aerospace, and various other fields requiring durable, transparent, and chemically resistant materials.
Beyond BPA: Alternative Monomers and Sustainability Concerns
While BPA remains the dominant monomer for Lexan production, there are ongoing efforts to explore alternative monomers and manufacturing processes that address concerns about BPA's potential endocrine-disrupting effects. Research is focused on finding monomers with comparable properties but a lower environmental impact. This includes investigating bio-based monomers and developing sustainable production methods. The shift towards sustainable materials and manufacturing practices is a key area of ongoing development within the polycarbonate industry.
Conclusion
Lexan polycarbonate is a remarkable polymer with exceptional properties derived from its fundamental building block: bisphenol A. The transesterification polymerization process meticulously crafts the long chains, resulting in a material with high impact resistance, optical clarity, and thermal stability. Its applications span various industries, highlighting its versatility and indispensable nature. However, the environmental considerations surrounding BPA are driving research into sustainable alternatives and manufacturing techniques. As the field continues to evolve, the future of Lexan and its production methods promises a balance between performance and sustainability.
Latest Posts
Latest Posts
-
Cellulose And Starch Are Examples Of
Mar 29, 2025
-
What Is Procedural History In A Case Brief
Mar 29, 2025
-
Is Sweating Negative Or Positive Feedback
Mar 29, 2025
-
Grinstead And Snell Introduction To Probability
Mar 29, 2025
-
What Is A Solution To An Equation
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Question Lexan Draw The Monomer Used To Make This Polymer . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
