Reduction Of An Aldehyde Produces A
Muz Play
Mar 19, 2025 · 5 min read
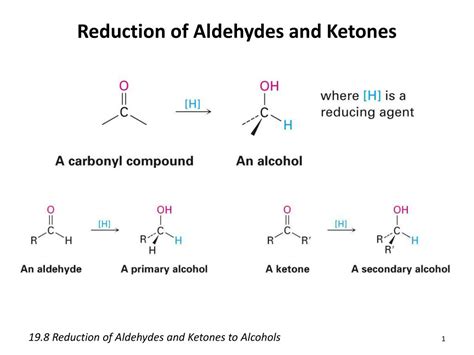
Table of Contents
Reduction of an Aldehyde Produces a Primary Alcohol: A Comprehensive Guide
The reduction of an aldehyde is a fundamental reaction in organic chemistry, resulting in the formation of a primary alcohol. This transformation is widely utilized in various synthetic pathways, offering a versatile tool for chemists to construct complex molecules. This comprehensive guide delves into the intricacies of aldehyde reduction, exploring different reducing agents, reaction mechanisms, and applications. We'll also touch upon important considerations for achieving high yields and selectivity.
Understanding Aldehydes and their Reduction
Aldehydes, characterized by a carbonyl group (C=O) bonded to at least one hydrogen atom, are readily susceptible to reduction. The carbonyl carbon, being electrophilic due to the polar nature of the C=O bond, is the target for nucleophilic attack by the reducing agent. This nucleophilic attack ultimately leads to the formation of a new C-H bond and the conversion of the carbonyl group into a hydroxyl (-OH) group.
The product of aldehyde reduction is always a primary alcohol, where the hydroxyl group is attached to a carbon atom that is bonded to only one other carbon atom. This characteristic differentiates it from secondary and tertiary alcohols formed by the reduction of ketones and other carbonyl compounds.
The Importance of Choosing the Right Reducing Agent
The success of an aldehyde reduction hinges on selecting the appropriate reducing agent. Different reducing agents offer varying degrees of selectivity and reactivity, making the choice dependent on the specific aldehyde substrate and desired reaction conditions. Some commonly employed reducing agents include:
-
Sodium Borohydride (NaBH₄): A mild reducing agent, NaBH₄ is widely used for reducing aldehydes and ketones in protic solvents like methanol or ethanol. It is relatively selective, avoiding the reduction of other functional groups present in the molecule. Its mild nature makes it ideal for substrates sensitive to harsh reaction conditions.
-
Lithium Aluminum Hydride (LiAlH₄): A much stronger reducing agent than NaBH₄, LiAlH₄ can reduce a wider range of carbonyl compounds, including esters, carboxylic acids, and amides. However, its strong reducing power often necessitates careful control of reaction conditions to prevent over-reduction or side reactions. It is typically used in anhydrous ether solvents.
-
Diborane (B₂H₆): This reagent is highly reactive and capable of reducing aldehydes to primary alcohols efficiently. Diborane is often generated in situ to avoid handling the gaseous and highly toxic diborane itself.
Reaction Mechanisms: A Deeper Dive
The mechanism of aldehyde reduction, regardless of the reducing agent employed, generally involves a nucleophilic attack on the carbonyl carbon. Let's examine the mechanism using sodium borohydride as an example:
-
Nucleophilic Attack: The hydride ion (H⁻) from NaBH₄ acts as a nucleophile, attacking the electrophilic carbonyl carbon of the aldehyde. This attack forms a tetrahedral intermediate.
-
Protonation: The negatively charged oxygen in the tetrahedral intermediate is then protonated by a proton source (e.g., methanol), leading to the formation of an alkoxide ion.
-
Protonation (again): A second protonation of the alkoxide ion occurs, yielding the primary alcohol as the final product. The boron-containing byproduct is then typically hydrolyzed to produce boric acid.
Detailed Mechanism using LiAlH₄
The mechanism with LiAlH₄ is similar but involves more complex interactions due to its greater reactivity. The hydride ion from LiAlH₄ attacks the carbonyl carbon, forming a tetrahedral intermediate. Subsequent protonation steps, often using a dilute acid workup, yield the primary alcohol. The aluminum-containing byproduct is then hydrolyzed.
Factors Affecting Reaction Yield and Selectivity
Several factors significantly influence the yield and selectivity of aldehyde reduction:
-
Solvent: The choice of solvent plays a crucial role in dissolving the reactants and facilitating the reaction. Protic solvents are commonly preferred for NaBH₄ reductions, while anhydrous ether solvents are typical for LiAlH₄.
-
Temperature: Reaction temperature impacts the reaction rate and selectivity. Lower temperatures often favor higher selectivity, preventing side reactions.
-
Steric Hindrance: Bulky substituents around the carbonyl group can hinder nucleophilic attack, affecting reaction rate and potentially yield.
-
Presence of Other Functional Groups: The presence of other reactive functional groups in the molecule can influence the outcome of the reaction. Careful selection of the reducing agent and reaction conditions is vital to achieve the desired selectivity.
Applications of Aldehyde Reduction in Organic Synthesis
Aldehyde reduction is a cornerstone reaction in various synthetic pathways, enabling the creation of a wide array of valuable compounds. Some notable applications include:
-
Synthesis of pharmaceuticals: Numerous pharmaceuticals contain alcohol functionalities derived from the reduction of aldehydes.
-
Production of fine chemicals: The reduction of aldehydes is vital in producing various fine chemicals used in industries like fragrances, flavors, and cosmetics.
-
Polymer chemistry: Alcohols synthesized via aldehyde reduction are frequently employed as monomers or building blocks in polymer synthesis.
-
Synthesis of natural products: Many natural products contain alcohol functional groups produced by reducing aldehydes found in nature.
Variations and Advanced Techniques
While the basic reduction of aldehydes is straightforward, several variations and advanced techniques exist to address specific challenges or achieve higher levels of control:
-
Catalytic Hydrogenation: This method uses a metal catalyst (e.g., palladium, platinum) and hydrogen gas to reduce aldehydes. It is a highly efficient and widely used technique, particularly for industrial-scale applications.
-
Enzymatic Reduction: Enzymes offer highly selective and environmentally friendly approaches to aldehyde reduction. This method is gaining increasing importance in green chemistry.
Conclusion
The reduction of an aldehyde to a primary alcohol is a fundamental and versatile transformation in organic chemistry. Understanding the reaction mechanism, choosing the appropriate reducing agent, and considering the various factors affecting yield and selectivity are crucial for successful execution. The widespread applications of this reaction highlight its significance in various fields, solidifying its place as an essential tool in the synthetic chemist's arsenal. Further exploration of the advanced techniques and variations discussed will enable chemists to optimize their synthesis strategies and achieve high efficiency and selectivity in their reactions. The continued advancements in this field promise even more sophisticated and efficient methods for aldehyde reduction in the future. The ever-growing need for sustainable and environmentally friendly synthetic methods will certainly drive further innovation in this crucial area of organic chemistry.
Latest Posts
Latest Posts
-
Solving Equations By Adding And Subtracting
Mar 19, 2025
-
Organisms That Gain Energy From Chemical Compounds Are Called
Mar 19, 2025
-
How To Find Electric Field From Electric Potential
Mar 19, 2025
-
What Is A Personal Exercise Programme
Mar 19, 2025
-
What Is A Ligand In Biology
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Reduction Of An Aldehyde Produces A . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
