S P D F Blocks On The Periodic Table
Muz Play
Mar 18, 2025 · 6 min read
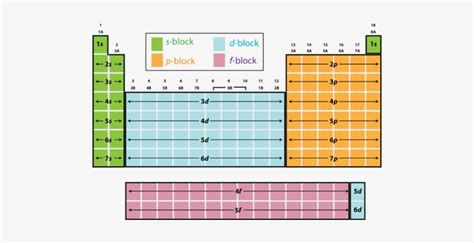
Table of Contents
Understanding the s, p, d, and f Blocks on the Periodic Table
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring chemical properties. While seemingly simple at first glance, the table holds a wealth of information, much of which is encoded within its organization into blocks: the s-block, p-block, d-block, and f-block. Understanding these blocks is crucial to grasping the fundamental behavior of elements and predicting their reactivity. This article will delve deep into each block, exploring their electronic configurations, characteristic properties, and the trends observed within them.
The s-Block: Alkali and Alkaline Earth Metals
The s-block elements occupy the first two columns of the periodic table. These elements are characterized by their valence electrons residing in the s orbital of their outermost electron shell. This leads to predictable and often reactive chemical behavior.
Group 1: Alkali Metals (Li, Na, K, Rb, Cs, Fr)
Alkali metals are highly reactive due to their single valence electron. This electron is easily lost, resulting in the formation of +1 ions. This tendency towards ionization explains their characteristic properties:
- Low ionization energies: Relatively little energy is required to remove the single valence electron.
- Low electronegativity: They have a weak attraction for electrons, making them readily donate their valence electron.
- Soft metals: They are easily cut with a knife.
- Low densities: They are lighter than most other metals.
- High reactivity: They react vigorously with water, producing hydrogen gas and metal hydroxides. For example, sodium reacts violently, while lithium reacts more moderately. The reactivity increases down the group.
Group 2: Alkaline Earth Metals (Be, Mg, Ca, Sr, Ba, Ra)
Alkaline earth metals possess two valence electrons in their outermost s orbital. While still relatively reactive, they are less so than alkali metals because removing two electrons requires more energy. Their properties include:
- Higher ionization energies than alkali metals: Removing two electrons requires more energy than removing one.
- Higher electronegativity than alkali metals: They have a stronger attraction for electrons compared to alkali metals.
- Harder and denser than alkali metals: They are less soft and have greater densities.
- Reactive with water (though less vigorously than alkali metals): Their reactivity also increases down the group, with beryllium showing minimal reactivity with water.
The p-Block: A Diverse Group of Elements
The p-block elements occupy the six columns to the right of the s-block. These elements fill their outermost p orbitals, resulting in a wide range of properties and applications. This block encompasses a variety of elements, including nonmetals, metalloids, and some metals.
Trends in the p-Block
Across a period (row) in the p-block, the electronegativity generally increases as the nuclear charge increases, pulling electrons closer to the nucleus. Down a group, electronegativity decreases as the atomic radius increases, leading to a weaker attraction between the nucleus and valence electrons.
Groups 13-18: Specific Properties
Each group within the p-block exhibits unique characteristics:
-
Group 13 (Boron Group): These elements possess three valence electrons and display a range of properties, from the metalloid boron to the metals aluminum, gallium, indium, and thallium. They form compounds with a +3 oxidation state.
-
Group 14 (Carbon Group): This group shows a significant variation in properties. Carbon is a nonmetal crucial to life, silicon and germanium are metalloids used in semiconductors, while tin and lead are metals. They exhibit varied oxidation states.
-
Group 15 (Nitrogen Group): This group contains nitrogen (a vital component of the atmosphere), phosphorus (essential for life), arsenic, antimony, and bismuth. They exhibit multiple oxidation states and show a transition from nonmetallic to metallic behavior down the group.
-
Group 16 (Oxygen Group or Chalcogens): Oxygen, sulfur, selenium, tellurium, and polonium are members of this group. Oxygen is essential for respiration, and sulfur has diverse applications in industry. The elements exhibit a -2 oxidation state in many compounds.
-
Group 17 (Halogens): Fluorine, chlorine, bromine, iodine, and astatine are highly reactive nonmetals with seven valence electrons. They readily gain one electron to form -1 ions. Their reactivity decreases down the group.
-
Group 18 (Noble Gases): Helium, neon, argon, krypton, xenon, and radon are inert gases with full valence electron shells (eight electrons, except for helium with two). Their stability makes them chemically unreactive under normal conditions.
The d-Block: Transition Metals
The d-block elements, also known as transition metals, occupy the central region of the periodic table. They are characterized by filling the d orbitals of the penultimate electron shell (the shell before the outermost shell). This results in a unique set of properties.
Characteristics of Transition Metals
-
Variable oxidation states: Transition metals can exhibit multiple oxidation states because of their ability to lose electrons from both the s and d orbitals. This leads to a rich variety of compounds with varying properties.
-
Formation of colored compounds: Many transition metal compounds are brightly colored due to the electronic transitions within the d orbitals. This is because the energy difference between these d orbitals is often in the range of visible light.
-
Catalytic activity: Many transition metals and their compounds act as catalysts in various chemical reactions. Their variable oxidation states and ability to form complexes allow them to facilitate chemical transformations.
-
Formation of complex ions: Transition metals readily form complex ions by coordinating with ligands (molecules or ions). These complexes play a crucial role in various biological processes and industrial applications.
-
Metallic properties: Transition metals typically exhibit high melting points, boiling points, and densities, characteristic of metals. They are also good conductors of heat and electricity.
Specific Properties of d-Block Groups
While a detailed exploration of each group is extensive, understanding the general trend of increasing atomic radius and decreasing electronegativity down a group and the opposite trend across a period is fundamental.
The f-Block: Inner Transition Metals (Lanthanides and Actinides)
The f-block elements, located at the bottom of the periodic table, are further divided into the lanthanides and actinides. These elements are characterized by filling the f orbitals.
Lanthanides (Rare Earth Elements)
The lanthanides are characterized by filling the 4f orbitals. They have very similar chemical properties due to the shielding effect of the 4f electrons.
Actinides
The actinides are characterized by filling the 5f orbitals. Unlike lanthanides, many actinides are radioactive and undergo various nuclear decays.
Properties of f-Block Elements
-
Similar chemical properties within each series: The lanthanides exhibit very similar chemical properties, making their separation challenging. A similar trend, though less pronounced, is observed in the actinides.
-
Radioactivity (Actinides): Most actinides are radioactive and many are highly radioactive, presenting significant challenges for handling and study.
-
Applications: Lanthanides and actinides have a range of applications in various technologies, from lighting and magnets (lanthanides) to nuclear energy (actinides).
Conclusion: A Unified Perspective
The s, p, d, and f blocks represent a powerful framework for understanding the organization and properties of elements on the periodic table. The periodic trends in atomic radius, ionization energy, electronegativity, and reactivity are directly related to the electronic configurations within these blocks. Understanding these blocks provides a fundamental basis for predicting the chemical behavior of elements and their role in various chemical and physical processes. From the highly reactive alkali metals to the inert noble gases, and from the versatile transition metals to the radioactive actinides, the periodic table’s block structure serves as a comprehensive roadmap to the vast and fascinating world of chemical elements. Further exploration of individual elements and their compounds within each block will reveal the intricacies and importance of the periodic table's organization in the study of chemistry.
Latest Posts
Latest Posts
-
What Is The Percentage Of Truth In A Joke
Mar 18, 2025
-
T Test Formula For Dependent Samples
Mar 18, 2025
-
Eriksons Stage Of Integrity Vs Despair
Mar 18, 2025
-
What Is The Difference Between Microscopic And Macroscopic
Mar 18, 2025
-
Determine The Degrees Of Freedom For The F Statistic
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about S P D F Blocks On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
