Standard Gibbs Free Energy Of Formation Table
Muz Play
Mar 22, 2025 · 7 min read
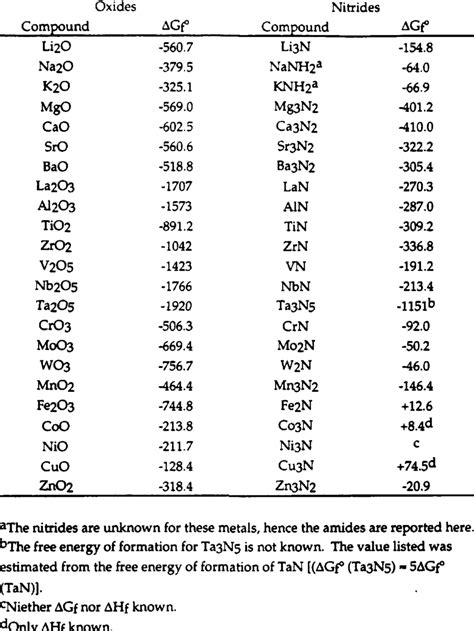
Table of Contents
Standard Gibbs Free Energy of Formation Table: A Comprehensive Guide
The standard Gibbs free energy of formation, denoted as Δ<sub>f</sub>G°, is a crucial thermodynamic property that dictates the spontaneity of a chemical reaction under standard conditions (298.15 K and 1 atm pressure). It represents the change in Gibbs free energy when one mole of a substance is formed from its constituent elements in their standard states. Understanding and utilizing a standard Gibbs free energy of formation table is essential for various applications in chemistry, chemical engineering, and materials science. This comprehensive guide will delve into the significance of Δ<sub>f</sub>G°, explore its applications, and provide insights into interpreting and using a standard Gibbs free energy of formation table effectively.
Understanding Gibbs Free Energy and its Formation
Before diving into the table itself, let's solidify our understanding of Gibbs free energy (G). Gibbs free energy is a thermodynamic potential that measures the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressure. It's a function of both enthalpy (H) and entropy (S), defined by the equation:
G = H - TS
Where:
- G is the Gibbs free energy
- H is the enthalpy (heat content)
- T is the absolute temperature
- S is the entropy (disorder)
The change in Gibbs free energy (ΔG) for a reaction determines its spontaneity:
- ΔG < 0: The reaction is spontaneous (exergonic) under the given conditions.
- ΔG > 0: The reaction is non-spontaneous (endergonic) under the given conditions. It requires energy input to proceed.
- ΔG = 0: The reaction is at equilibrium. There is no net change in the concentrations of reactants and products.
The standard Gibbs free energy of formation (Δ<sub>f</sub>G°) specifically refers to the change in Gibbs free energy when one mole of a compound is formed from its constituent elements in their standard states. The standard state for a substance is its most stable form at 298.15 K and 1 atm pressure. For example, the standard state of carbon is graphite, not diamond.
Interpreting a Standard Gibbs Free Energy of Formation Table
A standard Gibbs free energy of formation table lists the Δ<sub>f</sub>G° values for various substances. These tables typically organize data by chemical formula or compound name, providing the Δ<sub>f</sub>G° value in kJ/mol. The values are often presented at a standard temperature of 298.15 K (25°C). It's crucial to note that these values are relative; the Δ<sub>f</sub>G° of elements in their standard states is defined as zero.
Example of a Table Entry:
| Compound | Formula | Δ<sub>f</sub>G° (kJ/mol) |
|---|---|---|
| Water | H₂O(l) | -237.1 |
| Carbon Dioxide | CO₂(g) | -394.4 |
| Methane | CH₄(g) | -50.8 |
This excerpt shows that the formation of liquid water from its constituent elements (hydrogen and oxygen) releases 237.1 kJ/mol of Gibbs free energy, indicating a spontaneous process. Conversely, positive values would indicate a non-spontaneous formation process.
Applications of Standard Gibbs Free Energy of Formation
The standard Gibbs free energy of formation table has wide-ranging applications in various fields:
1. Predicting Reaction Spontaneity:
By using the Δ<sub>f</sub>G° values of reactants and products, one can calculate the standard Gibbs free energy change (Δ<sub>r</sub>G°) for a reaction using the following equation:
Δ<sub>r</sub>G° = Σ Δ<sub>f</sub>G°(products) - Σ Δ<sub>f</sub>G°(reactants)
A negative Δ<sub>r</sub>G° indicates a spontaneous reaction under standard conditions, while a positive Δ<sub>r</sub>G° suggests a non-spontaneous reaction. This is invaluable for predicting the feasibility of a chemical reaction.
2. Determining Equilibrium Constants:
The standard Gibbs free energy change is directly related to the equilibrium constant (K) of a reaction through the following equation:
Δ<sub>r</sub>G° = -RTlnK
Where:
- R is the ideal gas constant (8.314 J/mol·K)
- T is the absolute temperature
This allows for the calculation of the equilibrium constant, providing insight into the extent of the reaction at equilibrium.
3. Understanding Electrochemical Cells:
In electrochemistry, Δ<sub>f</sub>G° plays a vital role in determining the cell potential (E°) of an electrochemical cell:
Δ<sub>r</sub>G° = -nFE°
Where:
- n is the number of moles of electrons transferred in the reaction
- F is the Faraday constant (96485 C/mol)
This equation links the thermodynamic spontaneity (Δ<sub>r</sub>G°) to the electrical work that can be obtained from a reaction.
4. Materials Science and Engineering:
Δ<sub>f</sub>G° values are crucial in materials science for predicting the stability of different phases and compounds at various temperatures and pressures. This is vital for designing and selecting materials with desired properties.
5. Geochemistry and Environmental Science:
In geochemistry, the Δ<sub>f</sub>G° helps understand the formation and stability of minerals and rocks. In environmental science, it aids in predicting the fate of pollutants and the equilibrium of chemical species in natural systems.
Limitations and Considerations when using the Table
While incredibly useful, it's crucial to remember the limitations of a standard Gibbs free energy of formation table:
-
Standard Conditions: The values are valid only under standard conditions (298.15 K and 1 atm). Changes in temperature and pressure will affect the Gibbs free energy. More complex calculations are needed to account for non-standard conditions.
-
Ideal Behavior: The table assumes ideal behavior of gases and solutions. Deviations from ideality, especially at high concentrations, can significantly impact the accuracy of calculations.
-
Activity vs. Concentration: Strictly speaking, the equilibrium constant and Gibbs free energy equations should utilize activities instead of concentrations. However, at low concentrations, concentration can be a good approximation of activity.
-
Phase Transitions: The table might only list values for one phase of a substance (e.g., liquid water). Phase transitions (e.g., melting, boiling) must be considered when working with substances at temperatures other than 25°C.
-
Accuracy of Data: The accuracy of the Δ<sub>f</sub>G° values depends on the experimental methods used to determine them. Small errors in the experimental data can propagate through calculations, especially when dealing with multiple steps or reactions.
Expanding Your Understanding and Application
To effectively utilize a standard Gibbs free energy of formation table, consider these advanced points:
-
Temperature Dependence: The Gibbs free energy is temperature-dependent. Using the enthalpy and entropy changes of formation (Δ<sub>f</sub>H° and Δ<sub>f</sub>S°) allows the calculation of Δ<sub>f</sub>G° at different temperatures using the following equation:
Δ<sub>f</sub>G°(T) = Δ<sub>f</sub>H° - TΔ<sub>f</sub>S°
-
Non-Standard Conditions: To handle non-standard conditions, modify the Gibbs free energy using activities instead of concentrations, and appropriate correction factors to account for temperature and pressure changes.
-
Complex Reactions: For complex reactions involving multiple steps, the overall Δ<sub>r</sub>G° can be calculated by summing the individual Δ<sub>r</sub>G° values for each step.
-
Software and Databases: Several software packages and online databases provide extensive tables of thermodynamic data, including Δ<sub>f</sub>G° values, often with temperature dependence and improved accuracy.
Conclusion
The standard Gibbs free energy of formation table is an invaluable resource for chemists, engineers, and scientists across various disciplines. By understanding its principles, limitations, and applications, one can accurately predict reaction spontaneity, determine equilibrium constants, and gain insights into the behavior of chemical systems. Mastering this tool is essential for effective problem-solving and advanced thermodynamic analysis. Remember to always consider the limitations and strive for accurate data to obtain meaningful and reliable results. Continuous learning and exploration of more advanced thermodynamic concepts will significantly enhance your ability to leverage this important tool for deeper chemical insights.
Latest Posts
Latest Posts
-
Similarity Of Animal Cell And Plant Cell
Mar 23, 2025
-
How To Calculate Change In Temperature
Mar 23, 2025
-
What Is Gas Liquid Chromatography Used For
Mar 23, 2025
-
Formula For Average Value Of A Function
Mar 23, 2025
-
Type 1 And 2 Errors Examples
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Standard Gibbs Free Energy Of Formation Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
