The Elements In Group 1 Are Called The
Muz Play
Apr 04, 2025 · 6 min read
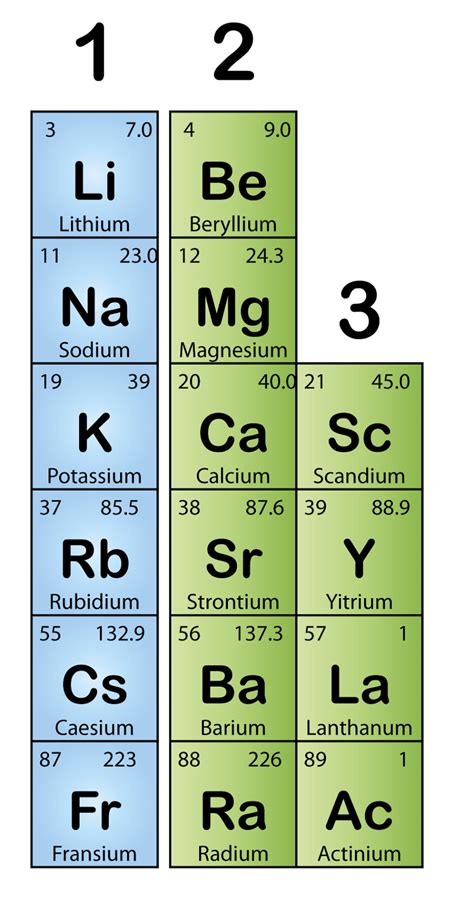
Table of Contents
- The Elements In Group 1 Are Called The
- Table of Contents
- The Elements in Group 1 are Called the Alkali Metals: A Deep Dive
- Defining Alkali Metals: Properties and Characteristics
- Electronic Configuration and Valence Electrons: The Key to Reactivity
- Low Ionization Energies: Easy Electron Loss
- Low Electronegativity: Preferring to Lose Electrons
- Physical Properties: Trends and Variations
- Chemical Reactions: Reactivity with Water, Oxygen, and Halogens
- Reaction with Water: A Vigorous Affair
- Reaction with Oxygen: Oxide Formation
- Reaction with Halogens: Salt Formation
- Trends in Alkali Metal Properties: Atomic Radius, Ionization Energy, and Electronegativity
- Atomic Radius: Increasing Size
- Ionization Energy: Decreasing Trend
- Electronegativity: Consistent Low Values
- Applications of Alkali Metals: From Batteries to Medicine
- Lithium-Ion Batteries: Powering Portable Devices
- Sodium in Streetlights and Lamps: Illuminating Our Cities
- Potassium in Fertilizers: Essential for Plant Growth
- Rubidium and Cesium in Atomic Clocks: Precision Timekeeping
- Medical Applications: Electrolyte Balance and Therapeutics
- Safety Precautions: Handling Alkali Metals with Care
- Conclusion: Understanding the Alkali Metals' Significance
- Latest Posts
- Latest Posts
- Related Post
The Elements in Group 1 are Called the Alkali Metals: A Deep Dive
The elements in Group 1 of the periodic table are collectively known as the alkali metals. This isn't just a catchy name; it reflects their unique chemical properties and reactivity. Understanding these elements is crucial for comprehending various aspects of chemistry, from basic chemical reactions to the intricate workings of biological systems and technological applications. This comprehensive guide will explore the alkali metals, delving into their characteristics, reactions, trends, and significant applications.
Defining Alkali Metals: Properties and Characteristics
The alkali metals – lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr) – share a distinct set of properties that define their group. These properties stem from their similar electronic configurations and the subsequent behavior of their outermost electrons.
Electronic Configuration and Valence Electrons: The Key to Reactivity
The defining characteristic of alkali metals is their electronic configuration. They all possess a single electron in their outermost (valence) shell. This single valence electron is relatively loosely held, making it readily available for participation in chemical reactions. This easy loss of an electron is what dictates their high reactivity.
Low Ionization Energies: Easy Electron Loss
Alkali metals have exceptionally low ionization energies. Ionization energy is the energy required to remove an electron from an atom. Because their outermost electron is loosely bound, it requires minimal energy to remove it, resulting in the formation of a +1 ion (cation). This ease of ionization is directly responsible for their high reactivity.
Low Electronegativity: Preferring to Lose Electrons
Electronegativity measures an atom's ability to attract electrons in a chemical bond. Alkali metals have very low electronegativity values. This means they are much more likely to lose their valence electron to another atom than to gain an electron. This preference for electron loss reinforces their tendency to form positive ions.
Physical Properties: Trends and Variations
While they share similar chemical properties, alkali metals also exhibit distinct physical properties that follow observable trends down the group.
- Melting and Boiling Points: Alkali metals have relatively low melting and boiling points compared to other metals. This is because the metallic bonding strength weakens as the atomic size increases down the group, requiring less energy to overcome these forces.
- Density: Density generally increases down the group, although it's important to note that even the densest alkali metal (cesium) is less dense than most other metals.
- Appearance: All alkali metals are silvery-white and lustrous when freshly cut, but they quickly tarnish in air due to their high reactivity with oxygen and moisture.
- Hardness: Alkali metals are extremely soft and can be easily cut with a knife. Their softness increases down the group due to weaker metallic bonding.
- Electrical Conductivity: They are excellent conductors of electricity, a property related to the ease of movement of their valence electrons.
Chemical Reactions: Reactivity with Water, Oxygen, and Halogens
The high reactivity of alkali metals is a defining feature. They readily react with various substances, especially water, oxygen, and halogens. Let's examine these reactions in detail:
Reaction with Water: A Vigorous Affair
The reaction of alkali metals with water is highly exothermic (releases a significant amount of heat) and often violent. The reaction produces hydrogen gas and the corresponding metal hydroxide. The reactivity increases dramatically down the group.
- Lithium (Li): Reacts relatively slowly with water, producing a gentle fizz.
- Sodium (Na): Reacts more vigorously, producing a more noticeable fizz and potentially igniting the hydrogen gas.
- Potassium (K): Reacts very rapidly, generating enough heat to ignite the hydrogen gas, resulting in a fiery reaction.
- Rubidium (Rb) and Cesium (Cs): These react explosively with water, posing a significant safety risk.
The general equation for the reaction is:
2M(s) + 2H₂O(l) → 2MOH(aq) + H₂(g)
Where M represents the alkali metal.
Reaction with Oxygen: Oxide Formation
Alkali metals readily react with oxygen in the air, forming metal oxides. The nature of the oxide formed varies depending on the metal and the conditions of the reaction. Lithium forms lithium oxide (Li₂O), while sodium primarily forms sodium peroxide (Na₂O₂), and potassium, rubidium, and cesium form superoxides (MO₂).
Reaction with Halogens: Salt Formation
Alkali metals react vigorously with halogens (Group 17 elements like chlorine, bromine, and iodine) to form ionic salts. These salts are crystalline solids with high melting points. The general reaction is:
2M(s) + X₂(g) → 2MX(s)
Where M represents the alkali metal and X represents the halogen. For example, sodium reacting with chlorine produces sodium chloride (NaCl), common table salt.
Trends in Alkali Metal Properties: Atomic Radius, Ionization Energy, and Electronegativity
As we move down Group 1, several important trends are observed in the alkali metals' properties:
Atomic Radius: Increasing Size
The atomic radius of alkali metals increases significantly down the group. This is because each subsequent element adds an additional electron shell, increasing the distance between the nucleus and the outermost electrons.
Ionization Energy: Decreasing Trend
As the atomic radius increases, the ionization energy decreases. The outermost electron is further away from the nucleus, experiencing weaker electrostatic attraction, making it easier to remove.
Electronegativity: Consistent Low Values
Electronegativity remains consistently low for all alkali metals. Although there is a slight decrease down the group, the values remain significantly lower than those of other elements, reinforcing their tendency to lose electrons.
Applications of Alkali Metals: From Batteries to Medicine
Alkali metals and their compounds have a wide range of applications across various industries and scientific fields:
Lithium-Ion Batteries: Powering Portable Devices
Lithium is a crucial component in lithium-ion batteries, powering our smartphones, laptops, electric vehicles, and many other portable electronic devices. Lithium's high electrochemical potential and low density make it ideal for this application.
Sodium in Streetlights and Lamps: Illuminating Our Cities
Sodium is used in sodium-vapor lamps, which provide bright, efficient lighting in streetlights and other outdoor applications.
Potassium in Fertilizers: Essential for Plant Growth
Potassium is an essential nutrient for plant growth and is a major component of many fertilizers. It plays a vital role in various plant metabolic processes.
Rubidium and Cesium in Atomic Clocks: Precision Timekeeping
Rubidium and cesium are used in atomic clocks, providing incredibly precise timekeeping, which is critical for various applications, including navigation systems and scientific research.
Medical Applications: Electrolyte Balance and Therapeutics
Sodium and potassium are crucial electrolytes in the human body, playing vital roles in maintaining fluid balance, nerve impulses, and muscle contractions. They are also essential components of various medications and intravenous solutions.
Safety Precautions: Handling Alkali Metals with Care
Due to their high reactivity, alkali metals pose significant safety risks. Direct contact with water or air can lead to violent reactions, causing burns and fires. Appropriate safety measures, including specialized handling techniques and protective equipment, must always be employed when working with these elements.
Conclusion: Understanding the Alkali Metals' Significance
The alkali metals are fascinating elements with unique properties and wide-ranging applications. Their high reactivity, stemming from their single valence electron, makes them crucial players in various chemical reactions and industrial processes. From powering our devices to providing essential nutrients for life, understanding the behavior and applications of alkali metals is fundamental to numerous scientific and technological advancements. Their unique chemical and physical properties provide a cornerstone for exploring the deeper principles of chemistry and their multifaceted roles in our modern world. Continued research and innovation promise even more exciting discoveries and applications of these reactive yet invaluable elements in the future.
Latest Posts
Latest Posts
-
How Big Is A Sheep Brain
Apr 08, 2025
-
What Generation Is Dominant In Ferns
Apr 08, 2025
-
What Is Pyruvic Acid Changed Into In Alcoholic Fermentation
Apr 08, 2025
-
Label The Brain Of The Sheep
Apr 08, 2025
-
An Organism That Obtains Energy By Feeding On Other Organisms
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about The Elements In Group 1 Are Called The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.