The Heisenberg Uncertainty Principle States That
Muz Play
Mar 27, 2025 · 6 min read
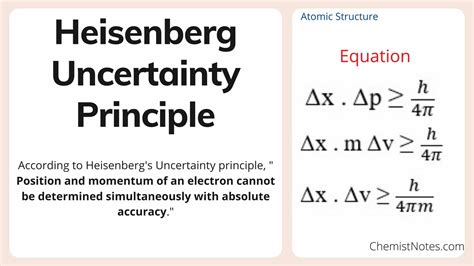
Table of Contents
The Heisenberg Uncertainty Principle: Delving into the Heart of Quantum Mechanics
The Heisenberg Uncertainty Principle, a cornerstone of quantum mechanics, dictates a fundamental limit to the precision with which certain pairs of physical properties of a particle, known as complementary variables, can be known simultaneously. This isn't simply a limitation of our measuring instruments; it's an inherent property of the universe at the quantum level. This principle profoundly impacts our understanding of the subatomic world and has far-reaching consequences for various fields of science.
Understanding the Principle: A Closer Look
The most common formulation of the uncertainty principle involves position (x) and momentum (p). It states that the product of the uncertainties in these two quantities is always greater than or equal to a constant, reduced Planck's constant (ħ, pronounced "h-bar"), divided by two:
Δx * Δp ≥ ħ/2
Where:
- Δx represents the uncertainty in position.
- Δp represents the uncertainty in momentum.
- ħ = h/2π where h is Planck's constant (approximately 6.626 x 10^-34 Js).
This inequality means that if we know the position of a particle with high precision (small Δx), then our knowledge of its momentum will be inherently limited (large Δp), and vice versa. It's not a matter of improving our measurement techniques; the inherent fuzziness is a fundamental aspect of quantum reality.
Beyond Position and Momentum: Other Complementary Variables
The uncertainty principle isn't restricted to position and momentum. Other pairs of complementary variables also exhibit this uncertainty relationship, including:
-
Energy (E) and Time (t): ΔE * Δt ≥ ħ/2. This implies that the more precisely we know a particle's energy, the less precisely we can know the time at which it possesses that energy. This is crucial in understanding processes like radioactive decay, where the lifetime of an unstable nucleus is inversely related to the uncertainty in its energy.
-
Angular momentum (L) and Angle (θ): ΔL * Δθ ≥ ħ/2. This relationship is less intuitive but equally important in understanding the behavior of rotating systems at the quantum scale.
The Philosophical Implications: Challenging Classical Determinism
The Heisenberg Uncertainty Principle fundamentally challenges the classical deterministic worldview. In classical physics, if we know the initial conditions of a system (position, velocity, etc.), we can, in principle, predict its future behavior with perfect accuracy. However, the uncertainty principle demonstrates that this is impossible at the quantum level. The inherent uncertainty in the initial conditions prevents us from making precise predictions about a particle's future trajectory.
This doesn't mean that quantum mechanics is random or chaotic. It means that the probabilities of different outcomes are governed by the wave function, which describes the quantum state of the particle. The uncertainty principle simply limits the extent to which we can know both the initial state and future evolution of that system simultaneously.
The Role of Observation: The Observer Effect
The uncertainty principle is often misunderstood to be solely about the limitations of measurement. The popular misconception is that the act of measuring a particle disturbs it, introducing the uncertainty. While measurement certainly plays a role, the uncertainty is inherent in the quantum state itself, irrespective of observation.
The act of measurement forces the wave function to "collapse" into a definite state. Before measurement, the particle exists in a superposition of states, meaning it has a probability of being in multiple states simultaneously. Measurement forces a selection of one specific state, and this collapse is what brings about the uncertainty in the complementary variable. However, the uncertainty was present before the measurement; the measurement merely reveals it.
Mathematical Formalism: A Deeper Dive
The mathematical foundation of the uncertainty principle lies in the non-commutativity of the quantum mechanical operators representing the complementary variables. For example, the position operator (x̂) and the momentum operator (p̂) do not commute, meaning that their order of application matters:
x̂p̂ - p̂x̂ = iħ
This commutation relation directly leads to the uncertainty principle. The mathematical derivation involves using the standard deviation of the operators to represent the uncertainties and applying the Cauchy-Schwarz inequality. This derivation demonstrates the inherent mathematical constraint arising from the non-commutativity of the operators and not merely a limitation of measurement.
Experimental Verification: Evidence from the Quantum Realm
The Heisenberg Uncertainty Principle is not merely a theoretical construct; it has been experimentally verified numerous times. Experiments involving electron diffraction and other quantum phenomena consistently demonstrate the limits imposed by the principle. The precision with which position and momentum can be measured is always in accordance with the inequality. These experimental validations strongly support the principle's validity and its fundamental role in quantum mechanics.
The Implications for Technology: Harnessing Quantum Effects
The uncertainty principle, though seemingly a limitation, has enabled technological advancements. Understanding this principle is crucial for developing technologies based on quantum phenomena:
Quantum Computing:
Quantum computers utilize the principles of superposition and entanglement to perform computations in ways impossible for classical computers. The uncertainty principle plays a crucial role in these processes, defining the limits of control and measurement in quantum systems.
Quantum Cryptography:
Quantum cryptography exploits the uncertainty principle to create highly secure communication systems. The very act of eavesdropping on a quantum communication channel inevitably introduces disturbances that can be detected, making quantum cryptography significantly more secure than classical cryptography.
Nanotechnology:
At the nanoscale, the uncertainty principle becomes increasingly significant. Understanding and controlling quantum effects is critical in designing and manipulating nanoscale devices and materials. The limitations imposed by the principle must be considered to create functional nanotechnology.
Misconceptions and Common Mistakes: Clearing Up Confusion
There are several common misconceptions surrounding the Heisenberg Uncertainty Principle. It's crucial to understand that the principle is not about:
- Measurement disturbance: While measurement plays a role, the uncertainty is inherent in the quantum state.
- Ignorance: It's not simply that we don't know the precise values, but rather that these values are fundamentally undefined.
- A limitation of technology: It's not that we can improve our measuring devices to circumvent the principle; it's an inherent feature of the universe.
Conclusion: A Foundation for Quantum Understanding
The Heisenberg Uncertainty Principle stands as a testament to the strange and counterintuitive nature of the quantum world. It challenges classical notions of determinism and predictability, demonstrating that at the subatomic level, the universe operates according to fundamentally different rules. However, far from being a limitation, this principle is a cornerstone of quantum mechanics, providing a framework for understanding the behavior of matter at the fundamental level and driving innovation in numerous technological fields. Understanding and appreciating the implications of this principle is essential for anyone seeking to delve into the fascinating realm of quantum physics.
Latest Posts
Latest Posts
-
Difference Between Simple Distillation And Fractional
Mar 30, 2025
-
In A Nonpolar Covalent Bond Electrons Are
Mar 30, 2025
-
Ion Channels That Are Always Open Are Called
Mar 30, 2025
-
What Directly Causes The Athenians To Hide In Their Homes
Mar 30, 2025
-
Differentiate Between Isometric And Isotonic Contractions
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about The Heisenberg Uncertainty Principle States That . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
