Three Structural Components Of An Rna Nucleotide Monomer
Muz Play
Mar 19, 2025 · 7 min read
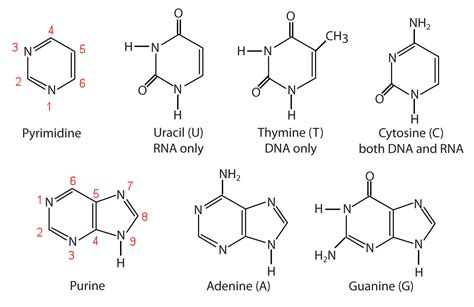
Table of Contents
Decoding the RNA Nucleotide: A Deep Dive into its Three Structural Components
Ribonucleic acid (RNA), a fundamental molecule in all living organisms, plays crucial roles in gene expression, protein synthesis, and various other cellular processes. Understanding RNA's structure is paramount to comprehending its multifaceted functions. At the heart of RNA's structure lies its monomeric unit: the RNA nucleotide. This article delves deep into the three essential structural components of an RNA nucleotide monomer, exploring their individual characteristics and their collective contribution to RNA's overall structure and function. We'll examine the intricacies of each component, highlighting their chemical properties and their impact on RNA's remarkable versatility.
1. The Sugar: Ribose – The Backbone of RNA Structure
The backbone of an RNA nucleotide, and indeed the entire RNA molecule, is formed by a five-carbon sugar molecule called ribose. This is a key distinguishing feature between RNA and DNA, which utilizes deoxyribose. The difference lies in the presence of a hydroxyl (-OH) group at the 2' carbon position in ribose. This seemingly small difference has profound implications for the structure and stability of the RNA molecule.
The 2'-Hydroxyl Group: A Key Differentiator
The presence of the 2'-hydroxyl group in ribose is responsible for several critical characteristics of RNA:
-
Increased Reactivity: The 2'-OH group makes ribose more chemically reactive than deoxyribose. This increased reactivity contributes to RNA's susceptibility to hydrolysis, meaning it's less stable than DNA. While this might seem like a disadvantage, this inherent instability is actually crucial for RNA's transient roles in many biological processes. The molecule's shorter lifespan ensures that it doesn't linger longer than necessary, preventing potential errors and allowing for efficient regulation of gene expression.
-
RNA's Structural Diversity: The 2'-OH group also influences RNA's ability to adopt a variety of secondary and tertiary structures. Unlike DNA, which primarily exists as a double helix, RNA can fold into complex three-dimensional structures, including hairpin loops, stem-loops, and pseudoknots. These intricate structures are essential for RNA's diverse functions, such as catalysis (ribozymes) and interaction with proteins. The 2'-OH group facilitates the formation of hydrogen bonds and other interactions that stabilize these complex structures.
-
Hydrolysis and RNA Degradation: The increased reactivity of ribose makes RNA prone to hydrolysis, particularly under alkaline conditions. The 2'-OH group can act as a nucleophile, attacking the phosphodiester bond that links adjacent nucleotides, leading to RNA degradation. This inherent instability is a crucial factor in the regulation of RNA lifespan and function within the cell.
Ribose's Cyclical Structure and Numbering
Ribose exists as a five-membered ring structure, a furanose ring. The carbon atoms within this ring are numbered 1' to 5', with the prime notation differentiating them from the atoms within the nitrogenous base. The numbering system is crucial for understanding the chemical linkages within the nucleotide and the overall RNA molecule. The 3' and 5' carbons are particularly important, as they are the points of attachment for the phosphate group that links nucleotides together to form the RNA polymer.
2. The Phosphate Group: Linking Nucleotides and Determining Charge
The phosphate group is another crucial component of the RNA nucleotide. It's a negatively charged group (PO43-) that connects the 3' carbon of one ribose sugar to the 5' carbon of the next ribose sugar. This forms the phosphodiester linkage, the backbone of the RNA polymer.
Phosphodiester Bond Formation: The RNA Polymer
The formation of the phosphodiester bond is a key step in RNA synthesis. This reaction involves the removal of a water molecule and creates a strong covalent bond between the phosphate group and the two ribose sugars. The negatively charged phosphate groups along the RNA backbone contribute significantly to the overall negative charge of the RNA molecule. This negative charge influences RNA's interactions with proteins and other molecules, playing a critical role in many biological processes.
Influence on RNA Conformation and Interactions
The phosphate backbone's negative charge contributes significantly to the RNA molecule's overall conformation and interactions with other molecules. This charge repulsion between phosphate groups influences the secondary and tertiary structures adopted by the RNA molecule. It also plays a vital role in interactions with positively charged proteins and metal ions, which often stabilize RNA's intricate three-dimensional structures and facilitate its various functions.
Phosphate's Role in Energy Transfer
While the primary role of the phosphate group within the RNA nucleotide is structural, it also contributes to the molecule's energy-related functions. In some cases, the phosphate group can act as an energy carrier, transferring energy during RNA synthesis and other cellular processes. The high-energy bonds within the phosphate group are essential for driving these reactions.
3. The Nitrogenous Base: Carrying Genetic Information
The third crucial component of an RNA nucleotide is the nitrogenous base. Unlike DNA, which uses four bases (adenine, guanine, cytosine, and thymine), RNA uses adenine (A), guanine (G), cytosine (C), and uracil (U). Uracil replaces thymine, differing only by the presence of a methyl group on thymine. These bases are attached to the 1' carbon of the ribose sugar via a glycosidic bond.
Purines and Pyrimidines: Their Structural Differences
The nitrogenous bases are categorized as either purines or pyrimidines. Purines (adenine and guanine) have a double-ring structure, whereas pyrimidines (cytosine and uracil) have a single-ring structure. This structural difference influences base stacking interactions within the RNA molecule and their ability to form hydrogen bonds with complementary bases.
Base Pairing and RNA Secondary Structure: A and U, G and C
The specific sequence of nitrogenous bases along the RNA molecule determines its primary structure. This sequence dictates how the RNA molecule folds into its secondary and tertiary structures. Base pairing is a crucial factor in determining RNA secondary structure. Adenine (A) forms two hydrogen bonds with uracil (U), and guanine (G) forms three hydrogen bonds with cytosine (C). These complementary base pairs are essential for the formation of hairpin loops, stem-loops, and other secondary structures that contribute to the RNA molecule's overall three-dimensional structure and function.
Uracil: A Unique Feature of RNA
The presence of uracil instead of thymine is a significant distinction between RNA and DNA. Uracil's lack of a methyl group makes it slightly more prone to chemical modification, adding another layer of complexity to RNA's function. This modification can influence RNA's stability, interactions with other molecules, and its regulatory role within the cell. The absence of the methyl group also allows for the easier degradation of uracil and therefore the RNA molecule itself.
The Nitrogenous Base's Role in RNA Function
The sequence of nitrogenous bases is critical for RNA's function. For example, messenger RNA (mRNA) carries genetic information from DNA to the ribosome, where it directs protein synthesis. Transfer RNA (tRNA) carries amino acids to the ribosome, and ribosomal RNA (rRNA) is a structural component of the ribosome. The specific sequence of bases in each of these RNA types is essential for their specific functions.
Conclusion: The Interplay of Components and RNA's Functionality
The three structural components of an RNA nucleotide—ribose, the phosphate group, and the nitrogenous base—work together in a remarkable interplay to create the diverse functionalities of RNA. The ribose sugar, with its reactive 2'-hydroxyl group, provides the backbone and influences RNA's structural flexibility and reactivity. The negatively charged phosphate group links the nucleotides, creating the overall negatively charged RNA backbone, which influences its interactions with other molecules. Finally, the nitrogenous bases, with their diverse base-pairing properties, carry the genetic information and are responsible for the intricate secondary and tertiary structures that drive RNA's many roles in the cell. Understanding these individual components and their synergistic interactions is essential for comprehending the full complexity and importance of RNA in life. Further research into the detailed interplay between these components will undoubtedly unveil new insights into the mechanisms of RNA-mediated biological processes.
Latest Posts
Latest Posts
-
What Is A Basic Unit Of Life
Mar 20, 2025
-
Ziegler Nichols Tuning Method For Pid Controller
Mar 20, 2025
-
Standard Free Energy Of Formation Table
Mar 20, 2025
-
R Showing All Entries As Singularity In Regression
Mar 20, 2025
-
Physical Or Chemical Change Ice Melting
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Three Structural Components Of An Rna Nucleotide Monomer . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
