Titration Curve Of Naoh And Hcl
Muz Play
Mar 31, 2025 · 6 min read
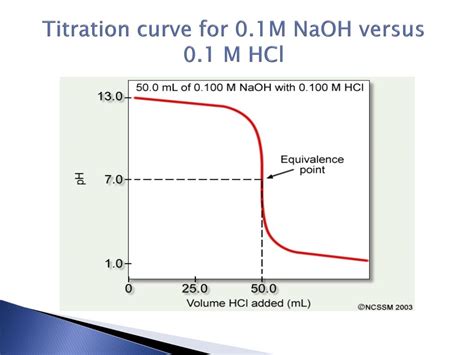
Table of Contents
Understanding the Titration Curve of NaOH and HCl: A Comprehensive Guide
The reaction between sodium hydroxide (NaOH), a strong base, and hydrochloric acid (HCl), a strong acid, is a classic example of a neutralization reaction. Understanding its titration curve is fundamental to grasping acid-base chemistry concepts. This comprehensive guide will delve into the intricacies of this titration, explaining the curve's shape, the significance of the equivalence point, and the factors influencing its characteristics. We will also explore how this knowledge applies to real-world applications.
The Neutralization Reaction: NaOH + HCl
The reaction between NaOH and HCl is a simple, fast, and complete neutralization reaction:
NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l)
This equation shows that one mole of NaOH reacts completely with one mole of HCl to produce one mole of sodium chloride (NaCl), a neutral salt, and one mole of water (H₂O). This 1:1 stoichiometry is crucial in interpreting the titration curve.
Constructing the Titration Curve
A titration curve is a graphical representation of the pH change of a solution as a titrant (in this case, NaOH) is added to an analyte (in this case, HCl). The x-axis typically represents the volume of titrant added, while the y-axis represents the pH of the solution. To understand the curve, let's break down its key regions:
1. Initial pH: Strong Acid Region
Before any NaOH is added, the solution contains only HCl. Since HCl is a strong acid, it completely dissociates in water:
HCl(aq) → H⁺(aq) + Cl⁻(aq)
The concentration of H⁺ ions directly determines the pH. Therefore, the initial pH will be relatively low, reflecting the high concentration of H⁺ ions. The exact value can be calculated using the formula:
pH = -log₁₀[H⁺]
where [H⁺] is the initial concentration of HCl.
2. Before the Equivalence Point: Buffer Region (Not Applicable Here)
In titrations involving weak acids or bases, a buffer region develops before the equivalence point. However, since both NaOH and HCl are strong, this region is absent. The pH increases gradually as NaOH is added, reflecting the neutralization of H⁺ ions.
3. The Equivalence Point: The Crucial Point
The equivalence point is the point in the titration where the moles of NaOH added equal the moles of HCl initially present. At this point, complete neutralization has occurred. Since NaCl is a neutral salt, the pH of the solution is 7.0. This is a key characteristic of strong acid-strong base titrations.
4. After the Equivalence Point: Strong Base Region
After the equivalence point, excess NaOH is present in the solution. NaOH is a strong base, completely dissociating into Na⁺ and OH⁻ ions. The excess OH⁻ ions determine the pH, which increases rapidly. The pH can be calculated using the concentration of the excess OH⁻ ions and the relationship:
pOH = -log₁₀[OH⁻]
and then using:
pH = 14 - pOH
The Shape of the Titration Curve: A Visual Representation
The titration curve for NaOH and HCl is characterized by a sharp, almost vertical rise in pH around the equivalence point. This steep rise reflects the rapid change in pH with the addition of a small volume of NaOH near the equivalence point. Before the equivalence point, the pH increases gradually. After the equivalence point, the pH increases more slowly, but the overall curve exhibits a sigmoidal shape.
Factors Influencing the Titration Curve
While the general shape remains consistent, several factors can subtly influence the precise details of the NaOH-HCl titration curve:
- Concentration of HCl: A higher initial concentration of HCl will result in a lower initial pH and a slightly less steep rise around the equivalence point. A lower concentration will have the opposite effect.
- Temperature: Temperature affects the dissociation constants of water and consequently the pH. Higher temperatures generally lead to slightly lower pH values.
- Ionic Strength: The presence of other ions in the solution can influence the activity coefficients of the ions involved, affecting the measured pH.
- Impurities: The presence of impurities in either the HCl or NaOH solutions can slightly alter the curve.
Applications of NaOH-HCl Titration
The NaOH-HCl titration, with its clear and easily interpreted curve, finds numerous applications in various fields:
- Acid-Base Analysis: This is the most direct application. The titration can be used to determine the concentration of an unknown acid or base solution by titrating it with a solution of known concentration.
- Industrial Quality Control: Various industries utilize acid-base titrations to ensure the quality of their products. For instance, the concentration of HCl in cleaning solutions or the amount of NaOH in food processing can be precisely determined.
- Environmental Monitoring: Acid-base titrations are vital for environmental monitoring to determine the acidity or alkalinity of water samples, assessing water quality and pollution levels.
- Chemical Synthesis: Titration is often used during chemical synthesis to monitor reaction progress and ensure optimal reaction conditions.
- Education: The NaOH-HCl titration serves as a fundamental experiment in chemistry education, illustrating key concepts of acid-base chemistry and titration techniques.
Choosing the Indicator: Phenolphthalein and Other Options
Choosing the right indicator is crucial for accurate determination of the equivalence point. Phenolphthalein is a commonly used indicator for strong acid-strong base titrations. It changes color from colorless to pink around pH 8.2-10.0, which falls within the sharp pH jump at the equivalence point of the NaOH-HCl titration.
Other indicators could be used, but they would need to have a color change range that encompasses the sharp pH change at the equivalence point. The choice of indicator depends on the specific titration and the desired accuracy.
Beyond the Basics: Understanding the Limitations
While the NaOH-HCl titration is straightforward, it’s important to acknowledge some limitations:
- Errors in Measurement: Errors in measuring the volumes of solutions can affect the accuracy of the results. Careful use of volumetric glassware is essential.
- Impurities: As mentioned earlier, impurities in the reagents can affect the titration results. Using high-purity reagents is crucial for accurate measurements.
- Equilibrium Considerations: While the reaction is rapid and essentially complete, at very low concentrations, equilibrium considerations might need to be taken into account.
Conclusion: A Powerful Tool in Chemistry
The titration curve of NaOH and HCl provides a clear and insightful illustration of the neutralization reaction between a strong acid and a strong base. Understanding the shape of the curve, the significance of the equivalence point, and the factors influencing its characteristics is fundamental for various chemical analyses. The simplicity and accuracy of this titration make it an invaluable tool in various fields, from industrial quality control to environmental monitoring and beyond. By mastering the principles behind this classic experiment, students and professionals alike can develop a deeper understanding of acid-base chemistry and its practical applications. This comprehensive guide aimed to provide a thorough understanding of the topic, highlighting both theoretical concepts and practical applications. Further exploration into the intricacies of acid-base chemistry will undoubtedly deepen one's appreciation for the power and elegance of this fundamental chemical reaction.
Latest Posts
Latest Posts
-
Is Table Salt Homogeneous Or Heterogeneous
Apr 02, 2025
-
What Part Of Bacteria Cell Helps It Move
Apr 02, 2025
-
Is The Organic Layer On The Top Or Bottom
Apr 02, 2025
-
In A Solution It Is Dissolving Medium
Apr 02, 2025
-
How To Find Moles Of Naoh Used In Titration
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Titration Curve Of Naoh And Hcl . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
