To Conduct Electricity A Solution Must Contain
Muz Play
Mar 18, 2025 · 6 min read
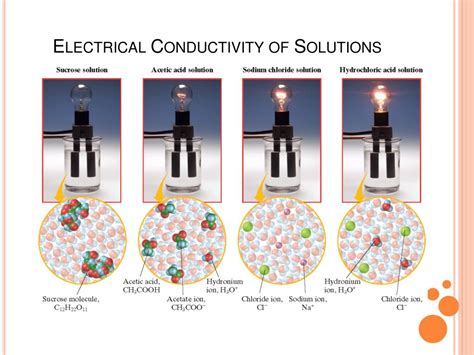
Table of Contents
To Conduct Electricity, a Solution Must Contain: Ions!
Electricity, the flow of electrons, is a fundamental force shaping our world. Understanding how it moves through different materials is crucial, especially when considering solutions – mixtures of a solute dissolved in a solvent. While pure water is a poor conductor, adding certain substances transforms it into a surprisingly effective electrical pathway. The key? Ions.
The Role of Ions in Electrical Conductivity
A solution's ability to conduct electricity hinges on the presence of charged particles, known as ions. These ions are atoms or molecules that have gained or lost electrons, resulting in a net positive or negative charge. When dissolved in a solvent, these ions become mobile, free to move and carry an electric current.
What are Ions?
- Cations: Positively charged ions formed when an atom loses electrons. Think of sodium (Na⁺) or potassium (K⁺) – common cations found in many solutions.
- Anions: Negatively charged ions formed when an atom gains electrons. Chloride (Cl⁻) and sulfate (SO₄²⁻) are examples of anions frequently encountered in conductive solutions.
These ions, unlike neutral molecules, can respond to an electric field. When a voltage is applied across a solution containing ions, the cations move towards the negative electrode (cathode), and the anions migrate towards the positive electrode (anode). This movement of charged particles constitutes an electric current.
Types of Solutions and Their Conductivity
Not all solutions conduct electricity equally well. The conductivity depends on several factors:
1. The Nature of the Solute:
-
Electrolytes: Substances that, when dissolved in a solvent, produce a solution that conducts electricity. These are typically ionic compounds, like salts (e.g., NaCl, table salt), acids (e.g., HCl, hydrochloric acid), and bases (e.g., NaOH, sodium hydroxide). Strong electrolytes completely dissociate into ions in solution, leading to high conductivity. Weak electrolytes partially dissociate, resulting in lower conductivity.
-
Non-electrolytes: Substances that do not dissociate into ions when dissolved. They do not conduct electricity. Examples include sugars (like sucrose) and alcohols (like ethanol). When these are dissolved, the molecules remain neutral and don't contribute to the charge carrier pool.
2. Concentration of the Solute:
The number of ions present in a solution directly impacts its conductivity. A higher concentration of electrolyte means more ions are available to carry the current, resulting in higher conductivity. This relationship is often not linear; at very high concentrations, ion-ion interactions can hinder conductivity.
3. The Nature of the Solvent:
The solvent's properties also influence conductivity. Water is a particularly good solvent for many ionic compounds because of its polarity. The polar nature of water molecules helps to stabilize the ions, promoting their dissociation and increasing conductivity. Other solvents, such as organic solvents, may be less effective at dissolving ionic compounds and therefore lead to lower conductivity.
4. Temperature:
Temperature plays a significant role in solution conductivity. Increasing temperature generally increases the kinetic energy of the ions, allowing them to move more freely and thus enhancing conductivity. This is because higher temperatures lead to increased collisions and dissociation of the solute.
Examples of Solutions that Conduct Electricity
Let's explore some everyday examples to illustrate the concept:
-
Saltwater: Dissolving table salt (NaCl) in water creates a highly conductive solution. Sodium (Na⁺) and chloride (Cl⁻) ions readily dissociate, allowing the easy flow of electricity. This is why you shouldn't use electrical appliances near water – the dissolved salts in the water can create a pathway for dangerous electrical currents.
-
Battery Acid: Automotive batteries utilize sulfuric acid (H₂SO₄) as an electrolyte. This strong acid dissociates into H⁺ and SO₄²⁻ ions, providing excellent conductivity necessary for the battery's operation.
-
Tap Water: While relatively pure water is a poor conductor, tap water contains dissolved minerals and salts, making it a weak electrolyte. The level of conductivity varies depending on the water source and mineral content.
-
Bodily Fluids: Our bodily fluids, such as blood and intracellular fluids, contain various ions like sodium, potassium, chloride, and calcium. These ions are essential for nerve impulse transmission and muscle contraction, processes that rely on electrical conductivity.
Applications of Conductive Solutions
The ability of solutions to conduct electricity has numerous applications:
-
Electroplating: This process uses conductive solutions to deposit a thin layer of metal onto an object. The solution contains ions of the desired metal, which are deposited onto the object's surface under the influence of an electric current.
-
Electrolysis: This technique uses electricity to drive non-spontaneous chemical reactions. Conductive solutions are crucial for providing a pathway for the electric current and allowing ions to participate in the redox reactions.
-
Batteries: Batteries rely on the movement of ions within an electrolyte solution to generate electricity. The electrolyte facilitates the flow of electrons between the electrodes, creating an electric current.
-
Sensors: Conductivity measurements can be used to monitor the concentration of ions in solutions, which has applications in various fields, from environmental monitoring to medical diagnostics.
Factors Affecting Conductivity: A Deeper Dive
Let's explore some of the factors affecting conductivity in more detail:
Degree of Ionization:
The extent to which a solute dissociates into ions directly impacts conductivity. Strong electrolytes, like most salts and strong acids/bases, fully ionize, while weak electrolytes only partially ionize, resulting in lower conductivity. Acetic acid (CH₃COOH), a weak acid, is a prime example. Only a small fraction of its molecules dissociate into acetate ions (CH₃COO⁻) and hydrogen ions (H⁺), leading to lower conductivity compared to a strong acid like hydrochloric acid (HCl).
Ionic Mobility:
The speed at which ions move through the solution under the influence of an electric field affects conductivity. Larger ions generally move slower than smaller ions due to increased frictional resistance from the solvent molecules. The viscosity of the solvent also plays a role; higher viscosity hinders ion movement, reducing conductivity.
Ion-Ion Interactions:
At higher concentrations, ions can interact with each other, forming ion pairs or clusters. These interactions reduce the number of free ions available to carry the current, thus lowering conductivity. This is known as the Debye-Hückel effect.
Solvent Properties:
The dielectric constant of the solvent plays a vital role. A higher dielectric constant means the solvent can better screen the electrostatic interactions between ions, promoting dissociation and increasing conductivity. Water has a high dielectric constant, making it an excellent solvent for ionic compounds.
Measuring Conductivity: Techniques and Applications
The conductivity of a solution is typically measured using a conductivity meter. This device measures the resistance of the solution to the flow of an electric current. The inverse of resistance is conductance, and conductivity is often expressed as specific conductance (Siemens per meter, S/m) or conductivity (μS/cm).
Conductivity measurements have diverse applications:
-
Water Quality Monitoring: Conductivity provides a quick indication of the total dissolved solids (TDS) in water, reflecting its purity and suitability for various purposes.
-
Industrial Process Control: Conductivity monitoring is essential in industrial processes to ensure the consistency and quality of products.
-
Medical Diagnostics: Conductivity measurements are used in medical settings to analyze bodily fluids and assess electrolyte balance.
-
Environmental Monitoring: Conductivity measurements aid in assessing water quality in rivers, lakes, and oceans, helping monitor pollution levels and environmental health.
Conclusion: The Importance of Ions in Electrical Conductivity
In summary, the ability of a solution to conduct electricity rests fundamentally on the presence of mobile ions. The type of solute, its concentration, the solvent's properties, temperature, and ion-ion interactions all contribute to the overall conductivity. Understanding these factors is essential across various scientific disciplines and industrial applications, from electrochemistry and material science to environmental monitoring and medical diagnostics. The ubiquitous presence of conductive solutions and the key role of ions underscore their importance in our daily lives and technological advancements.
Latest Posts
Latest Posts
-
What Is The Difference Between Primary And Secondary Growth
Mar 18, 2025
-
Work Done By An Electric Field
Mar 18, 2025
-
How Much Energy To Be At Zero Kinetic Energy
Mar 18, 2025
-
Current As A Function Of Time
Mar 18, 2025
-
Ode To Billy Joe Lyrics Meaning
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about To Conduct Electricity A Solution Must Contain . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
