Unit Expressing A Ratio Of A Solute In A Solution
Muz Play
Mar 20, 2025 · 6 min read
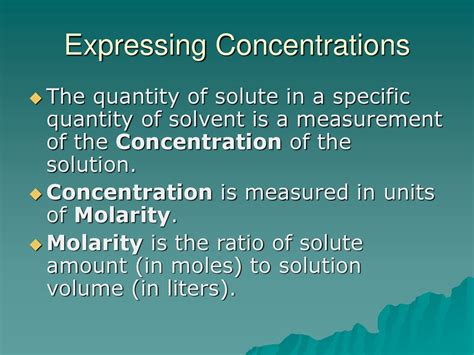
Table of Contents
Unit Expressing a Ratio of a Solute in a Solution: A Comprehensive Guide
Understanding how to express the concentration of a solute within a solution is fundamental in various scientific disciplines, from chemistry and biology to environmental science and pharmacology. Accurate representation of these ratios is crucial for consistent experimental results, proper dosage calculations, and effective communication of findings. This comprehensive guide delves into the numerous units used to express the ratio of a solute in a solution, exploring their definitions, applications, advantages, and disadvantages.
Understanding Solutions and Concentration
Before diving into the units, let's establish a clear understanding of the terminology. A solution is a homogeneous mixture consisting of a solute (the substance being dissolved) and a solvent (the substance doing the dissolving). The concentration of a solution describes the amount of solute present in a given amount of solution or solvent. Expressing concentration accurately is essential for reproducibility and meaningful interpretation of experimental data.
Common Units for Expressing Solute Concentration
Numerous units are employed to represent solute concentration, each offering unique advantages and disadvantages depending on the application. We'll explore some of the most commonly used units, categorizing them for clarity:
Mass-Based Units
These units express the mass of solute relative to the mass or volume of the solution or solvent.
-
Percent by Mass (% w/w): This unit expresses the mass of solute (in grams) per 100 grams of solution. It's straightforward and widely used, particularly in everyday applications and some industrial contexts. Example: A 10% w/w NaCl solution contains 10 grams of NaCl in 100 grams of solution (90 grams of water + 10 grams NaCl).
- Advantages: Easy to understand and calculate.
- Disadvantages: Temperature-dependent; the volume of the solution can change with temperature, affecting the concentration. Not suitable for very dilute solutions.
-
Parts per Million (ppm) and Parts per Billion (ppb): Used for extremely dilute solutions, ppm represents the mass of solute per million parts of solution (1 ppm = 1 mg/kg or 1 µg/g), and ppb represents the mass of solute per billion parts (1 ppb = 1 µg/kg or 1 ng/g). These are commonly employed in environmental monitoring and toxicology.
- Advantages: Suitable for extremely low concentrations.
- Disadvantages: Can be confusing due to the wide range of units it can represent (mg/kg, µg/g, etc.).
Volume-Based Units
These units relate the volume of the solute to the volume of the solution.
-
Percent by Volume (% v/v): This expresses the volume of solute (in milliliters) per 100 milliliters of solution. Frequently used when both solute and solvent are liquids. Example: A 70% v/v ethanol solution contains 70 ml of ethanol in 100 ml of solution.
- Advantages: Simple to understand and calculate, particularly useful for liquid-liquid solutions.
- Disadvantages: Temperature-dependent; volume changes with temperature.
-
Molarity (M): This is perhaps the most commonly used concentration unit in chemistry. It represents the number of moles of solute per liter of solution. Example: A 1 M NaCl solution contains 1 mole of NaCl dissolved in 1 liter of solution.
- Advantages: Directly relates to the number of solute particles, crucial for stoichiometric calculations.
- Disadvantages: Temperature-dependent due to volume changes.
-
Molality (m): Molality expresses the number of moles of solute per kilogram of solvent. Unlike molarity, it's independent of temperature. Example: A 1 m NaCl solution contains 1 mole of NaCl in 1 kg of water.
- Advantages: Temperature-independent, useful in colligative property calculations.
- Disadvantages: Requires precise measurement of solvent mass. Less convenient than molarity for many routine laboratory procedures.
Other Units
-
Normality (N): This expresses the number of gram equivalents of solute per liter of solution. It is less commonly used now compared to molarity, due to its dependence on the chemical reaction being studied.
- Advantages: Useful for acid-base titrations and redox reactions.
- Disadvantages: Reaction-dependent; not consistently applicable to all types of reactions. Less intuitive than molarity.
-
Mole Fraction (χ): The mole fraction of a component in a solution is the ratio of the number of moles of that component to the total number of moles of all components in the solution. Useful in thermodynamic calculations.
- Advantages: Temperature-independent, useful in thermodynamic calculations.
- Disadvantages: Less intuitive than other units, requires knowledge of the moles of all solution components.
-
Mass Concentration (ρ): This is often expressed in units like grams per liter (g/L) or kilograms per cubic meter (kg/m³). It signifies the mass of the solute per unit volume of solution.
- Advantages: Direct measure of solute mass per volume, relatively straightforward to use.
- Disadvantages: Temperature-dependent due to changes in solution volume.
Choosing the Appropriate Unit
The selection of an appropriate concentration unit hinges on the specific context and intended application. Consider the following factors:
- Accuracy Required: For highly precise work, units like molarity or molality are preferred over percent compositions.
- Temperature Dependence: If temperature fluctuations are anticipated, molality is a better choice than molarity or volume-based units.
- Nature of Solute and Solvent: Volume-based units are suitable for liquid-liquid solutions; mass-based units are more applicable to solutions where the solute is a solid.
- Intended Use: For stoichiometric calculations, molarity is essential. For environmental monitoring, ppm or ppb might be more practical.
Conversion Between Units
Frequently, it’s necessary to convert between different concentration units. This involves applying appropriate conversion factors based on the relationship between the units. For example, converting between molarity and percent by mass requires knowledge of the molar mass of the solute and the density of the solution. These conversions often involve a series of calculations and necessitate careful attention to unit consistency.
Practical Applications
The units discussed find extensive applications in numerous fields:
- Pharmaceutical Industry: Precise concentration is crucial for drug formulation and dosage calculations. Molarity and mass-based units are commonly used.
- Environmental Science: Monitoring pollutants in water and air requires units like ppm and ppb.
- Chemical Engineering: Molarity and molality are vital for process design and optimization.
- Food Science: Percent compositions are frequently used to label food products.
- Clinical Chemistry: Concentration units are essential for analyzing blood and other bodily fluids.
Conclusion
Expressing the ratio of a solute in a solution accurately is paramount for consistent experimental results and meaningful interpretations. Numerous units exist, each with strengths and limitations. Selecting the most appropriate unit depends on the specific application, requiring careful consideration of factors like accuracy, temperature dependence, and the nature of the solution components. Understanding these units and their interconversion is essential for success in various scientific and technological fields. With the appropriate knowledge and careful calculations, the precise concentration of any solute can be reliably represented and applied across a broad spectrum of scientific and practical applications.
Latest Posts
Latest Posts
-
How To Determine Ph With Molarity
Mar 21, 2025
-
What Is A Post Hoc Analysis
Mar 21, 2025
-
Why Des Concentraion Cause Blue To Green
Mar 21, 2025
-
What Is The Zone Of Inhibition
Mar 21, 2025
-
Chamber That Houses The Developing Fetus
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Unit Expressing A Ratio Of A Solute In A Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
