Using E-z Designators Identify The Configuration
Muz Play
Mar 17, 2025 · 6 min read
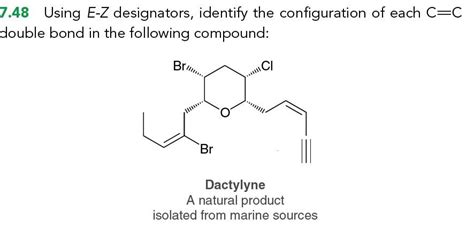
Table of Contents
Using E-Z Designators to Identify Configuration: A Comprehensive Guide
Understanding and utilizing E-Z designators is crucial for accurately identifying and communicating the configuration of chiral molecules. This system, based on the Cahn-Ingold-Prelog (CIP) priority rules, provides a standardized and unambiguous way to describe the three-dimensional arrangement of atoms around a chiral center. This comprehensive guide will delve into the intricacies of E-Z designators, explaining the process of assigning priorities, determining configuration, and navigating common scenarios.
Understanding Chirality and Stereoisomers
Before diving into E-Z notation, it's essential to grasp the fundamental concepts of chirality and stereoisomers. Chirality refers to the property of a molecule that is not superimposable on its mirror image. Such molecules are called chiral, and their mirror images are called enantiomers. These enantiomers have identical physical properties (except for how they interact with plane-polarized light) but can have vastly different biological activities.
Stereoisomers, in general, are isomers that have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms. Enantiomers are a type of stereoisomer. Another type is diastereomers, which are stereoisomers that are not mirror images of each other. E-Z notation specifically addresses a subset of stereoisomers: those with double bonds.
The Cahn-Ingold-Prelog (CIP) Priority Rules
The cornerstone of E-Z designation lies in the CIP priority rules. These rules dictate the order of priority assigned to substituents attached to a double bond. The higher the atomic number, the higher the priority. Here's a breakdown:
1. Atomic Number:
The first step is to compare the atomic numbers of the atoms directly bonded to the double-bonded carbons. The atom with the higher atomic number receives higher priority. For example, a bromine atom (Br, atomic number 35) has higher priority than a chlorine atom (Cl, atomic number 17).
2. Isotopes:
If the atoms directly bonded are isotopes of the same element, the heavier isotope gets higher priority. For example, deuterium (²H) has higher priority than protium (¹H).
3. Multiple Bonds:
Multiple bonds are treated as if they were multiple single bonds to that atom. For instance, a carbon atom double-bonded to another carbon is treated as if it were bonded to two separate carbon atoms. A triple bond is treated as if it were three single bonds.
4. Working Down the Chain:
If the atoms directly bonded are the same, you must move down the chain, comparing the atoms bonded to those atoms until a difference in priority is found. This process continues until a difference is identified.
Assigning E and Z Configurations
Once the priorities have been assigned to each substituent on both carbons of the double bond, the E-Z configuration can be determined.
-
E (entgegen): If the higher priority substituents on each carbon are on opposite sides of the double bond, the configuration is designated as E. Think of "E" as meaning "opposite."
-
Z (zusammen): If the higher priority substituents on each carbon are on the same side of the double bond, the configuration is designated as Z. Think of "Z" as meaning "together."
Illustrative Examples:
Let's examine some examples to solidify the understanding of E-Z designation:
Example 1: Simple Alkenes
Consider the molecule 1-chloropropene. One carbon of the double bond is attached to a chlorine atom (higher priority) and a hydrogen atom (lower priority). The other carbon is attached to a methyl group (CH3) and a hydrogen atom. Using the CIP rules, chlorine gets higher priority than hydrogen on one carbon, and the methyl group gets higher priority than hydrogen on the other carbon. Since the higher priority substituents (chlorine and methyl) are on opposite sides of the double bond, the configuration is E-1-chloropropene.
Example 2: Complex Substituents
Consider a molecule with more complex substituents. Let's imagine a double bond with one carbon bonded to a -COOH group and a -CH2CH3 group and the other carbon bonded to a -Br and a -CH3 group.
First we assign priorities:
- For the first carbon, the -COOH group has priority over the -CH2CH3 group.
- For the second carbon, the -Br has priority over the -CH3 group.
Since the higher priority groups (-COOH and -Br) are on the same side of the double bond, the configuration is Z.
Example 3: Dealing with Multiple Bonds
Consider a molecule with a double bond to an aldehyde group. The carbon of the double bond has one bond to hydrogen (H) and one to an aldehyde group (-CHO). The aldehyde carbon is doubly bonded to oxygen (O). Using the "multiple bonds as multiple single bonds" rule, the aldehyde carbon is treated as being bonded to two oxygen atoms. This results in the aldehyde group having higher priority than the hydrogen.
Example 4: Cyclic Compounds
The principles remain the same when dealing with cyclic compounds containing a double bond. The priority rules are applied to the substituents on the double-bonded carbons within the ring structure.
Practical Applications and Importance of E-Z Designators
The unambiguous nature of E-Z notation is crucial in various fields:
-
Organic Chemistry: Accurate communication of molecular structures is paramount in research, synthesis, and analysis. E-Z designators prevent ambiguity and ensure that researchers worldwide are working with the same molecule.
-
Pharmaceutical Industry: Many pharmaceuticals are chiral molecules, and the specific configuration often determines their biological activity and potential side effects. Precise identification using E-Z nomenclature is crucial for drug development and regulation.
-
Materials Science: The properties of materials often depend on the stereochemistry of their constituent molecules. E-Z designation helps characterize and control material properties.
-
Chemical Databases: E-Z notation is a standardized method used in databases to record and search for molecules with specific configurations.
Advanced Considerations:
Several nuances arise in more complex scenarios:
-
Multiple Double Bonds: If a molecule contains multiple double bonds, each must be assigned its individual E or Z configuration.
-
Rings and Cyclic Systems: Similar rules apply, but priority assignment may require carefully considering the atoms within the ring structure.
-
Unsaturated Systems: The CIP rules must be applied meticulously to ensure accurate priority assignments in molecules with multiple bonds and branched substituents.
Conclusion:
Mastering the use of E-Z designators requires a thorough understanding of the CIP priority rules and their application to different molecular structures. While the process may initially appear complex, consistent practice and careful attention to detail will solidify your understanding. The ability to accurately assign E and Z configurations is a fundamental skill for anyone working in fields involving organic chemistry, pharmaceuticals, or materials science. Accurate and unambiguous communication through E-Z notation is critical for scientific rigor and successful collaboration. By diligently applying these principles, you contribute to the clear and accurate representation of molecular structures. Remember to always check and double check your assignments!
Latest Posts
Latest Posts
-
A Relationship In Which Both Organisms Benefit
Mar 18, 2025
-
If One Of The Reactants In A Reaction Is
Mar 18, 2025
-
Is Melting Point Physical Or Chemical Property
Mar 18, 2025
-
Find The Arclength Of The Curve
Mar 18, 2025
-
Which One Leaves The Solution Untouched
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Using E-z Designators Identify The Configuration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
