Water Is Always A Product In What Type Of Reaction
Muz Play
Mar 23, 2025 · 5 min read
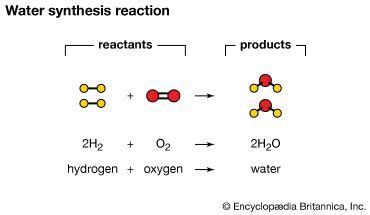
Table of Contents
Water is Always a Product in What Type of Reaction?
Water, the elixir of life, plays a crucial role in countless chemical reactions. Understanding its involvement, particularly when it's a product, is key to comprehending various chemical processes. This article delves deep into the types of reactions where water is consistently formed, exploring the underlying mechanisms and providing illustrative examples. We'll also touch upon the significance of these reactions in different fields, from biological processes to industrial applications.
Water as a Product: The Significance of Synthesis Reactions
The most common reaction type where water is invariably produced is a synthesis reaction, specifically a condensation reaction. In these reactions, two or more simpler molecules combine to form a larger, more complex molecule, with the simultaneous release of a water molecule. This process is often called dehydration synthesis because water is essentially removed during the formation of the larger molecule.
Understanding Condensation Reactions
Condensation reactions are fundamentally about the formation of a new bond between two reacting molecules. This bond formation is accompanied by the elimination of a water molecule. The water molecule is formed by the combination of a hydroxyl group (-OH) from one reactant and a hydrogen atom (H) from the other.
Example 1: Formation of a Disaccharide
Consider the formation of sucrose (table sugar) from glucose and fructose. Both glucose and fructose are monosaccharides (simple sugars). When they combine, a glycosidic bond forms between them, releasing a water molecule in the process. This is a classic example of dehydration synthesis resulting in a disaccharide.
Example 2: Peptide Bond Formation
The synthesis of proteins involves the formation of peptide bonds between amino acids. During this reaction, a water molecule is eliminated as the carboxyl group (-COOH) of one amino acid reacts with the amino group (-NH2) of another. The resulting peptide bond connects the two amino acids. This process is essential for protein synthesis in all living organisms.
Example 3: Esterification
Esterification is another significant type of condensation reaction where an ester is formed from the reaction of a carboxylic acid and an alcohol. A water molecule is removed as the bond between the acid and alcohol is formed, resulting in the characteristic ester functional group. Esters are commonly found in fragrances, flavors, and fats.
Beyond Condensation: Other Reaction Types Yielding Water
While condensation reactions are the most prominent examples, water can also be formed as a byproduct in other reaction types. Let's explore some less common scenarios:
Combustion Reactions: A Significant Source of Water
The complete combustion of organic compounds (hydrocarbons) in the presence of sufficient oxygen produces carbon dioxide and water. This is a highly exothermic reaction, releasing significant amounts of energy. The water produced is a consequence of the oxidation of hydrogen atoms present in the hydrocarbon molecule.
Example: Combustion of Methane
The combustion of methane (CH₄), the primary component of natural gas, follows the equation:
CH₄ + 2O₂ → CO₂ + 2H₂O
Here, the hydrogen atoms in methane react with oxygen to form water molecules. This reaction is crucial in power generation and various industrial processes.
Neutralization Reactions: Water as a Product of Acid-Base Reactions
When a strong acid reacts with a strong base, a neutralization reaction occurs. The product of this reaction is always salt and water. The hydrogen ions (H⁺) from the acid combine with the hydroxide ions (OH⁻) from the base to form water.
Example: Reaction between Hydrochloric Acid and Sodium Hydroxide
HCl (aq) + NaOH (aq) → NaCl (aq) + H₂O (l)
This reaction is a classic example of a neutralization reaction, where the strong acid (HCl) and the strong base (NaOH) react to produce the salt sodium chloride (NaCl) and water.
Hydration Reactions: Water as a Reactant and Product
Hydration reactions involve the addition of water to a molecule. While water is a reactant, it can also be a product in specific instances. For instance, the hydration of an alkene can lead to an alcohol, but subsequent reactions of that alcohol might release water again. The net production or consumption of water depends on the specific reaction pathway.
Hydrolysis Reactions: The Reverse of Condensation
Hydrolysis reactions are essentially the reverse of condensation reactions. They involve the breaking of a bond in a molecule by the addition of water. This process often requires a catalyst, such as an acid or an enzyme. Water is consumed in the process, not produced. However, depending on the context, subsequent reactions might release water.
The Importance of Water Formation in Various Fields
The formation of water as a product in chemical reactions has far-reaching implications across numerous fields:
Biological Systems: The Foundation of Life
The condensation reactions involved in the synthesis of biomolecules, such as proteins, carbohydrates, and nucleic acids, are vital for life itself. The release of water during these reactions is essential for building the complex structures that make up living organisms.
Industrial Applications: Energy Production and Chemical Synthesis
Combustion reactions are crucial for energy production in power plants and internal combustion engines. The production of water in these reactions is a natural consequence of the process. Furthermore, numerous industrial chemical processes involve condensation reactions, resulting in the formation of water as a byproduct.
Environmental Significance: Water Cycle and Climate Change
The water cycle relies on the continuous exchange of water between the atmosphere, land, and oceans. Chemical reactions, including combustion and biological processes, contribute significantly to the water cycle. Understanding these reactions is essential for analyzing the impact of human activities on the environment and climate change.
Conclusion: Water – A Universal Byproduct of Creation and Change
Water's consistent appearance as a product in many chemical reactions highlights its fundamental role in chemistry and biology. Condensation reactions, combustion, and neutralization reactions are prime examples where water formation is a defining feature. Understanding these reaction types and their implications is crucial for advancements in various fields, from developing new materials and energy sources to comprehending the intricacies of life itself. The ubiquitous nature of water and its involvement in these reactions underscore its crucial position in the intricate tapestry of chemical and biological processes. Further exploration of specific reactions and their mechanisms offers a deeper understanding of the chemical world and the role water plays in shaping it.
Latest Posts
Latest Posts
-
Difference Between Pulmonary Circulation And Systemic Circulation
Mar 25, 2025
-
What Unusual Step Did Oregon Take To Increase Voter Registration
Mar 25, 2025
-
How To Factor Trinomials With A Coefficient
Mar 25, 2025
-
Identifying Reaction Types And Balancing Equations
Mar 25, 2025
-
Geography Of The Industrial Revolution Map
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Water Is Always A Product In What Type Of Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
