What Affects The Rate Of Diffusion
Muz Play
Mar 25, 2025 · 6 min read
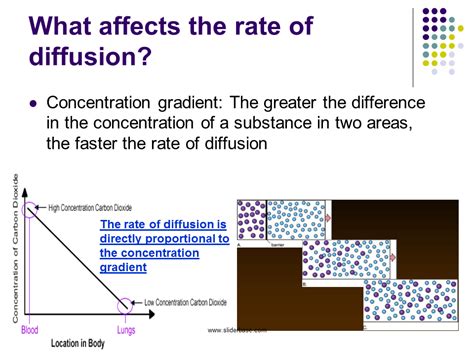
Table of Contents
What Affects the Rate of Diffusion? A Comprehensive Guide
Diffusion, the net movement of particles from a region of higher concentration to a region of lower concentration, is a fundamental process in numerous biological and physical systems. Understanding the factors that influence the rate of diffusion is crucial in various fields, from understanding cellular processes to designing efficient industrial separation techniques. This comprehensive guide delves into the key factors affecting diffusion rates, explaining the underlying mechanisms and providing practical examples.
The Fick's First Law of Diffusion: A Foundation
Before diving into the factors, it's important to establish a foundational understanding. Fick's First Law of Diffusion describes the rate of diffusion as being directly proportional to the concentration gradient and the diffusion coefficient. Mathematically, it's represented as:
J = -D (dC/dx)
Where:
- J represents the diffusion flux (the amount of substance diffusing per unit area per unit time).
- D is the diffusion coefficient (a measure of how readily a substance diffuses through a medium).
- dC/dx represents the concentration gradient (the change in concentration over distance). The negative sign indicates that diffusion occurs down the concentration gradient.
This law highlights that a steeper concentration gradient leads to a faster diffusion rate. However, the diffusion coefficient, D, is itself influenced by several crucial factors.
Key Factors Affecting the Rate of Diffusion
Let's now explore the primary factors that significantly impact the rate of diffusion:
1. Concentration Gradient: The Driving Force
The steeper the concentration gradient, the faster the rate of diffusion. Imagine dropping a dye tablet into a glass of water. Initially, the dye is highly concentrated near the tablet. This creates a large concentration gradient, resulting in rapid diffusion. As the dye spreads, the gradient lessens, and the diffusion rate slows down until equilibrium is reached – where the dye is evenly distributed.
Example: The rapid uptake of oxygen by the lungs is facilitated by a large concentration gradient between the air in the alveoli and the oxygen-depleted blood.
2. Temperature: Kinetic Energy's Role
Higher temperatures lead to increased kinetic energy of the particles. This means particles move faster and collide more frequently, leading to a higher rate of diffusion. The increased kinetic energy overcomes the intermolecular forces, allowing for easier movement of particles.
Example: Sugar dissolves faster in hot water than in cold water due to the higher kinetic energy of water molecules at elevated temperatures.
3. Mass of the Diffusing Particles: Size Matters
Larger, heavier particles diffuse more slowly than smaller, lighter ones. This is because larger particles possess higher inertia, resisting changes in motion. Therefore, they require more energy to move and diffuse.
Example: The diffusion of small gas molecules, like oxygen and carbon dioxide, is significantly faster compared to the diffusion of large protein molecules within a cell.
4. Medium of Diffusion: Viscosity and Porosity
The medium through which diffusion occurs significantly affects its rate. A more viscous medium, like honey, offers greater resistance to the movement of particles, slowing down diffusion. Conversely, a less viscous medium, like water, allows for faster diffusion.
Porosity also plays a role, particularly in solid materials. A porous material with interconnected spaces allows particles to diffuse more readily compared to a dense, non-porous material.
Example: Diffusion of gases through air is faster than diffusion through water because air is less viscous. The diffusion of water through soil is faster in loose, sandy soils compared to dense clay soils due to differences in porosity.
5. Surface Area: More Space, Faster Diffusion
A larger surface area available for diffusion enhances the rate. This is because more particles can simultaneously cross the boundary between regions of different concentrations.
Example: The highly folded structure of the small intestine lining increases the surface area available for nutrient absorption through diffusion. Similarly, the finely divided structure of catalysts increases their surface area for efficient chemical reactions.
6. Distance of Diffusion: The Barrier Effect
The distance over which diffusion occurs directly impacts the rate. Diffusion slows down significantly as the distance increases. This is because particles have to travel further, encountering more obstacles and collisions along the way.
Example: The efficient transport of oxygen from the lungs to the tissues depends on the relatively short distances involved. Diffusion over large distances is inefficient and often requires assistance from other transport mechanisms, such as convection.
7. Pressure Gradient: Driving Force in Gases
In the context of gases, a pressure gradient acts as an additional driving force for diffusion. Gases diffuse from a region of higher pressure to a region of lower pressure. The greater the pressure difference, the faster the rate of diffusion.
Example: The diffusion of air into the lungs is driven by the pressure difference between the atmosphere and the alveolar air.
8. Solubility of the Diffusing Substance: Dissolving Matters
The solubility of a substance in the medium plays a role, especially when considering the diffusion of gases or liquids into a liquid or solid medium. A substance that is more soluble in the medium will diffuse more readily.
Example: The solubility of carbon dioxide in blood is significantly influenced by factors like pH and temperature. This influences the rate of CO2 diffusion from tissues into the bloodstream.
Applications and Implications of Understanding Diffusion Rates
Understanding the factors affecting diffusion rates has numerous applications across diverse fields:
- Medicine: Drug delivery systems are designed to optimize diffusion rates to ensure effective medication absorption.
- Environmental Science: Understanding pollutant diffusion in soil and water is critical for environmental remediation strategies.
- Food Science: Food preservation techniques often involve controlling diffusion rates to prevent spoilage.
- Materials Science: The design and development of new materials often requires control over diffusion rates to achieve desired properties.
- Cellular Biology: Understanding diffusion in cell membranes is crucial for comprehending various physiological processes, including nutrient uptake and waste removal.
Beyond Fick's Law: Complexities and Nuances
While Fick's First Law provides a fundamental framework for understanding diffusion, it's essential to recognize that it simplifies reality. In many real-world scenarios, diffusion processes are more complex and involve other factors not explicitly captured by the law. These include:
- Non-uniform media: Diffusion in heterogeneous environments can be significantly more complicated due to varying properties of the medium.
- Interactions between diffusing particles: Attractive or repulsive forces between particles can alter diffusion rates.
- Coupled diffusion: The diffusion of one substance can influence the diffusion of another.
- Diffusion in confined spaces: Diffusion within narrow channels or pores can deviate from Fick's Law.
Conclusion: A Dynamic Process
Diffusion is a dynamic process influenced by a multitude of interdependent factors. While Fick's Law offers a basic understanding, appreciating the complexities and nuances associated with diffusion rates is crucial for accurate modeling and prediction in various scientific and engineering disciplines. By carefully considering the concentration gradient, temperature, mass of particles, medium properties, surface area, distance, pressure gradient, and solubility, a comprehensive understanding of diffusion can be achieved. This understanding empowers scientists, engineers, and researchers to optimize processes and design novel technologies across diverse fields.
Latest Posts
Latest Posts
-
What Are Three Characteristic Properties Of Ionic Compounds
Mar 25, 2025
-
Vice Of Excess And Deficiency Virtue Chart Scrupulosity Laxity
Mar 25, 2025
-
How Many Are In A Set
Mar 25, 2025
-
Biology Word That Starts With J
Mar 25, 2025
-
What Is The Instrument To Measure Humidity
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Affects The Rate Of Diffusion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
