What Are 3 Characteristics Of All Metals
Muz Play
Apr 06, 2025 · 6 min read
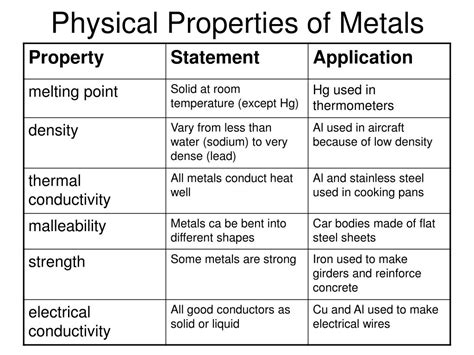
Table of Contents
What Are 3 Characteristics of All Metals? Delving Deep into Metallic Properties
Metals are ubiquitous in our daily lives, from the smartphones in our pockets to the skyscrapers that dominate our skylines. This pervasive presence highlights their crucial role in modern society. But what fundamentally defines a metal? While a vast array of metals exists, exhibiting a wide spectrum of properties, three core characteristics unite them all: high electrical conductivity, high thermal conductivity, and metallic bonding. Let's explore each of these in detail, uncovering the underlying reasons for their existence and their implications for the diverse applications of metals.
1. High Electrical Conductivity: The Flow of Electrons
One of the most defining characteristics of metals is their exceptional ability to conduct electricity. This property stems directly from the unique nature of metallic bonding, which we will discuss more thoroughly in the next section. Essentially, in metals, the valence electrons—the outermost electrons in an atom—are not tightly bound to individual atoms. Instead, they form a "sea" or "cloud" of delocalized electrons that are free to move throughout the entire metal structure.
Understanding Electron Mobility
This delocalized electron sea is the key to metallic conductivity. When an electric field is applied across a metal, these free electrons are readily accelerated in the direction of the field. This movement of charge constitutes an electric current. The greater the number of free electrons and the easier they can move through the lattice structure, the higher the electrical conductivity.
Conductivity Variations Among Metals
While all metals are good conductors, their conductivity varies significantly depending on factors such as:
- Purity: Impurities in a metal disrupt the regular arrangement of atoms and hinder the movement of electrons, thus reducing conductivity. Highly pure metals generally exhibit higher conductivity.
- Temperature: Increased temperature leads to increased vibrations of the metal atoms, making it more difficult for electrons to navigate the lattice. Therefore, the conductivity of metals typically decreases with increasing temperature.
- Crystal Structure: The arrangement of atoms in a metal's crystal structure influences electron mobility. Certain crystal structures offer more efficient pathways for electron flow than others.
Practical Implications of High Electrical Conductivity
The high electrical conductivity of metals is exploited extensively in various applications, including:
- Electrical wiring: Copper and aluminum are widely used in electrical wiring due to their excellent conductivity and relatively low cost.
- Electronics: Metals like gold, silver, and copper are essential components in electronic circuits and devices due to their high conductivity and resistance to corrosion.
- Power transmission: High-voltage power lines often utilize aluminum due to its lightweight nature and good conductivity.
2. High Thermal Conductivity: Efficient Heat Transfer
Closely related to electrical conductivity is the high thermal conductivity of metals. Just as electrons move freely to conduct electricity, they also efficiently transfer thermal energy (heat) through the metal. When one part of a metal is heated, the increased kinetic energy of the atoms and free electrons is rapidly transferred to adjacent atoms and electrons, leading to a quick distribution of heat throughout the material.
The Role of Free Electrons in Heat Transfer
The free electrons in the metallic lattice play a crucial role in heat transfer. These electrons, constantly moving and colliding with atoms, effectively transport thermal energy from hotter to colder regions of the metal. This mechanism explains why metals feel cold to the touch—they rapidly absorb heat from your hand.
Comparing Thermal Conductivities
Again, while all metals exhibit high thermal conductivity, the specific value varies considerably depending on factors similar to those influencing electrical conductivity:
- Purity: Impurities scatter phonons (quantized vibrations of the lattice) and electrons, reducing thermal conductivity.
- Temperature: Increased temperature typically reduces thermal conductivity, though the effect is more complex than in electrical conductivity and depends on the specific metal.
- Crystal Structure: The crystal structure plays a significant role in determining how efficiently phonons and electrons can transport heat.
Applications Leveraging High Thermal Conductivity
The high thermal conductivity of metals finds numerous applications in areas where efficient heat transfer is critical:
- Heat sinks: Metals like copper and aluminum are used extensively in heat sinks to dissipate heat from electronic components.
- Cooking utensils: Metals such as stainless steel and copper are common in cookware due to their ability to distribute heat evenly.
- Heat exchangers: Metals are vital components in heat exchangers used in various industrial processes and power generation.
3. Metallic Bonding: The Foundation of Metallic Properties
The fundamental reason behind the high electrical and thermal conductivities of metals lies in their unique type of chemical bonding: metallic bonding. Unlike ionic or covalent bonds, where electrons are localized between specific atoms, metallic bonding involves a "sea" of delocalized electrons surrounding a lattice of positively charged metal ions.
Delocalized Electrons: The Key to Metallic Bonding
In a metallic solid, the valence electrons are not associated with any particular atom; instead, they are free to move throughout the entire metal structure. This creates a "sea" of electrons that acts as a glue, holding the positively charged metal ions together. The strength of the metallic bond depends on factors such as the number of valence electrons and the size of the metal ions.
The "Electron Sea" Model
The "electron sea" model provides a simple yet effective way to visualize metallic bonding. Imagine a lattice of positively charged metal ions immersed in a sea of freely moving electrons. These electrons are not bound to any specific ion, allowing for their facile movement and contributing to the characteristic properties of metals.
Variations in Metallic Bonding Strength
While the basic principle of metallic bonding remains consistent across all metals, the strength of the bond varies depending on factors such as:
- Number of valence electrons: Metals with more valence electrons generally have stronger metallic bonds.
- Atomic radius: Smaller atoms typically form stronger metallic bonds due to increased electrostatic attraction between the nuclei and the electron sea.
- Electron configuration: The specific electron configuration influences the ease with which electrons delocalize and thus affects bond strength.
Consequences of Metallic Bonding
The nature of metallic bonding directly leads to many of the characteristic properties of metals, including:
- Malleability and Ductility: The delocalized electrons allow metal atoms to slide past each other without disrupting the metallic bonding, making metals malleable (easily shaped) and ductile (easily drawn into wires).
- Luster: The free electrons interact with light, giving metals their characteristic metallic luster or shine.
- High Density: The close packing of metal atoms in the lattice contributes to their relatively high density.
- Opacity: Metals generally appear opaque due to the absorption of light by the delocalized electrons.
Conclusion: The Uniqueness of Metallic Properties
The three characteristics—high electrical conductivity, high thermal conductivity, and metallic bonding—are fundamental to the nature of metals. These properties are inextricably linked, stemming from the unique arrangement of atoms and the behavior of electrons in metallic solids. Understanding these fundamental properties is crucial for appreciating the wide-ranging applications of metals in various fields, from electronics and construction to transportation and energy production. The remarkable versatility of metals is a direct consequence of their fundamental metallic nature. Further exploration into specific types of metals and their specialized properties will reveal an even richer understanding of this essential class of materials.
Latest Posts
Latest Posts
-
Combine The Sentences Into One Sentence
Apr 08, 2025
-
How Do You Count Bacterial Colonies
Apr 08, 2025
-
Osmosis Involves Which Type Of Membrane Transport
Apr 08, 2025
-
Do All Cells Come From Preexisting Cells
Apr 08, 2025
-
Where Do The Electrons Entering Photosystem Ii Come From
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about What Are 3 Characteristics Of All Metals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.