What Are The Blocks In The Periodic Table
Muz Play
Mar 28, 2025 · 7 min read
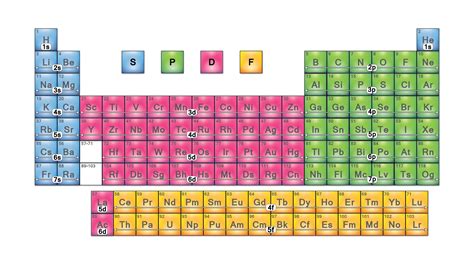
Table of Contents
What Are the Blocks in the Periodic Table? A Deep Dive into s, p, d, and f Blocks
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring chemical properties. While the arrangement itself is visually striking, a deeper understanding lies in the blocks that categorize elements based on the subshells where their valence electrons reside. Understanding these blocks—s, p, d, and f—is crucial for predicting an element's behavior, its reactivity, and its place within the broader landscape of chemical interactions. This comprehensive guide delves into each block, exploring their unique characteristics, representative elements, and overall significance in the world of chemistry.
Understanding Electron Configuration and the Blocks
Before diving into each block, it's essential to grasp the concept of electron configuration. This describes how electrons are arranged within the various energy levels and subshells of an atom. Each subshell is designated by a letter (s, p, d, f) and can hold a specific number of electrons:
- s subshell: Holds a maximum of 2 electrons.
- p subshell: Holds a maximum of 6 electrons.
- d subshell: Holds a maximum of 10 electrons.
- f subshell: Holds a maximum of 14 electrons.
The periodic table's blocks are defined by the highest energy subshell that contains valence electrons—the electrons involved in chemical bonding. These valence electrons are crucial in determining an element's chemical properties.
The s-Block Elements: Alkali Metals and Alkaline Earth Metals
The s-block occupies the far left of the periodic table, encompassing Groups 1 and 2. This block is characterized by elements whose valence electrons occupy the s subshell.
Group 1: Alkali Metals (excluding Hydrogen)
Alkali metals, including lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr), are highly reactive metals. Their single valence electron in the s subshell is easily lost, forming 1+ ions. This makes them highly electropositive and prone to vigorous reactions with water and other substances. Their reactivity generally increases down the group.
- Key characteristics: Low density, low melting points, excellent electrical conductivity, readily form ionic compounds.
- Examples of use: Sodium is crucial in table salt (NaCl), while potassium is vital for biological functions. Lithium finds application in batteries.
Group 2: Alkaline Earth Metals
Alkaline earth metals, including beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra), possess two valence electrons in their s subshell. They are less reactive than alkali metals but still readily lose their two valence electrons to form 2+ ions.
- Key characteristics: Higher density and melting points than alkali metals, good conductors of electricity, essential for biological processes.
- Examples of use: Magnesium is lightweight and strong, used in alloys, while calcium is a vital component of bones and teeth.
The p-Block Elements: A Diverse Group
The p-block occupies the right side of the periodic table, encompassing Groups 13 to 18. Elements in this block have valence electrons occupying the p subshell. This block exhibits remarkable diversity in properties, ranging from metals to nonmetals and metalloids.
Group 13: Boron Group
The boron group, including boron (B), aluminum (Al), gallium (Ga), indium (In), and thallium (Tl), shows a trend from metalloid to metal down the group. Boron is a metalloid, while the others are metals. They generally form 3+ ions.
- Key characteristics: Varying properties from metalloid to metal, relatively low melting points (except boron).
- Examples of use: Aluminum is widely used in construction and packaging, while boron is used in semiconductors.
Group 14: Carbon Group
The carbon group, including carbon (C), silicon (Si), germanium (Ge), tin (Sn), and lead (Pb), showcases a transition from nonmetal (carbon) to metalloid (silicon and germanium) to metal (tin and lead). Carbon is unique due to its ability to form extensive chains and networks, leading to the immense diversity of organic compounds.
- Key characteristics: Diverse properties, varying bonding capabilities, crucial for life (carbon).
- Examples of use: Silicon is essential in semiconductors and computer chips, while carbon forms the basis of all organic life.
Group 15: Nitrogen Group (Pnictogens)
The nitrogen group, including nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), and bismuth (Bi), shows a progression from nonmetal to metalloid to metal. Nitrogen is a crucial component of the atmosphere and biological molecules.
- Key characteristics: Varied properties, ability to form multiple bonds.
- Examples of use: Nitrogen is vital for fertilizers, while phosphorus is essential for biological systems and matches.
Group 16: Oxygen Group (Chalcogens)
The oxygen group, including oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and polonium (Po), includes oxygen, essential for respiration. This group displays increasing metallic character down the group.
- Key characteristics: Essential elements, diverse oxidation states, importance in biological systems.
- Examples of use: Oxygen is vital for respiration, while sulfur is used in vulcanizing rubber.
Group 17: Halogens
The halogens, including fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At), are highly reactive nonmetals. They readily gain one electron to form 1- ions, creating stable halide ions.
- Key characteristics: Highly reactive, strong oxidizing agents, form diatomic molecules.
- Examples of use: Chlorine is used in water purification, while iodine is essential for thyroid function.
Group 18: Noble Gases
Noble gases, including helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn), are exceptionally unreactive due to their full valence shells. This makes them chemically inert.
- Key characteristics: Inert, stable electron configuration, used in lighting and other applications.
- Examples of use: Helium is used in balloons and cryogenics, while neon is used in neon signs.
The d-Block Elements: Transition Metals
The d-block occupies the central region of the periodic table, encompassing Groups 3 to 12. These are the transition metals, known for their variable oxidation states and the formation of colorful compounds. Their valence electrons occupy the d subshell.
- Key characteristics: Variable oxidation states, formation of complex ions, catalytic activity, metallic properties.
- Examples of use: Iron (Fe) is crucial for steel production, while copper (Cu) is used in electrical wiring and plumbing. Many transition metals are essential catalysts in industrial processes. Platinum (Pt) and palladium (Pd) are widely used as catalysts in automotive catalytic converters and chemical reactions.
The f-Block Elements: Inner Transition Metals
The f-block elements are situated at the bottom of the periodic table, separated into two rows: the lanthanides (rare earth elements) and the actinides. These elements have valence electrons occupying the f subshell.
Lanthanides
The lanthanides, from cerium (Ce) to lutetium (Lu), are chemically similar due to the gradual filling of the 4f subshell.
- Key characteristics: Similar chemical properties, paramagnetic, used in various alloys and catalysts.
- Examples of use: Used in various alloys for their unique magnetic and electronic properties.
Actinides
The actinides, from thorium (Th) to lawrencium (Lr), are all radioactive. Many are synthetic elements.
- Key characteristics: Radioactive, heavy metals, used in nuclear reactors and research.
- Examples of use: Uranium (U) and plutonium (Pu) are used in nuclear reactors and weapons.
Conclusion: The Importance of Understanding the Blocks
Understanding the s, p, d, and f blocks of the periodic table is fundamental to comprehending the behavior and properties of elements. This knowledge allows chemists to predict reactivity, bonding patterns, and the myriad ways elements interact with each other, forming the basis for countless chemical reactions and applications across various fields, from medicine and materials science to energy production and environmental technologies. The periodic table is more than just a chart; it's a roadmap to the fundamental building blocks of our world, and understanding its blocks is key to unlocking its secrets.
Latest Posts
Latest Posts
-
The Building Blocks Of Nucleic Acids Are
Mar 31, 2025
-
Why A Cells Size Is Limited
Mar 31, 2025
-
Bones Of The Lower Limb Diagram
Mar 31, 2025
-
Explain How The Skin Helps In Regulating Body Temperature
Mar 31, 2025
-
Mannitol Salt Agar Selective Or Differential
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Are The Blocks In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
