What Are The Rows Of A Periodic Table Called
Muz Play
Mar 15, 2025 · 6 min read
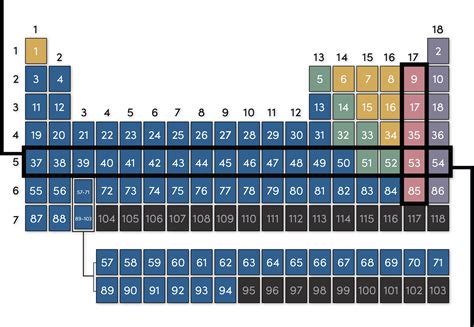
Table of Contents
What are the Rows of a Periodic Table Called? A Deep Dive into Periods and Their Significance
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. While many are familiar with the columns, known as groups or families, the horizontal rows also hold significant importance and are called periods. Understanding periods is crucial for grasping the trends and patterns in elemental properties. This article delves deep into what periods are, how they're organized, their significance, and the underlying principles that govern their arrangement.
Understanding Periods: A Horizontal Journey Through Elements
Periods in the periodic table represent the principal energy levels or electron shells that are being filled as you move across the row. Each period starts with an element that has a new outermost electron shell and ends with a complete outermost electron shell. This means that all elements within a given period have the same number of electron shells, though the number of electrons within those shells will vary.
Period 1: The Pioneers of the Table
The first period, remarkably short, contains only two elements: hydrogen (H) and helium (He). These elements fill the first electron shell, which can hold a maximum of two electrons. Hydrogen, with one electron, readily forms bonds to achieve stability, while helium, with a full electron shell, is inert and incredibly stable. This sets the stage for the more complex periods to follow.
Period 2 and 3: Building Blocks of Matter
Periods 2 and 3, consisting of eight elements each, showcase the filling of the second and third electron shells. These shells can accommodate up to eight electrons. We start to see trends emerge here, with elements exhibiting increasing electronegativity and ionization energy as we move across the period from left to right. The transition from metals to non-metals within these periods clearly demonstrates the periodic nature of elemental properties. This period provides the foundation for understanding the subsequent periods' properties and behaviors.
Period 4 and 5: Transitioning to Complexity
Period 4 and 5 each have 18 elements, a significant increase from the previous periods. This is due to the addition of the d subshell, capable of holding ten electrons. This addition leads to the introduction of the transition metals, characterized by their variable oxidation states and often colorful compounds. The transition metals display a fascinating range of properties and play crucial roles in various catalytic processes and biological systems. The expanded number of elements highlights the increasing complexity of electron shell filling and the subsequent changes in chemical behavior.
Period 6 and 7: Lanthanides, Actinides, and the Limits of Stability
Periods 6 and 7 are the longest, each containing 32 elements. This dramatic increase results from the inclusion of the f subshell, which can accommodate fourteen electrons. The elements associated with this subshell are the lanthanides (rare earth elements) in period 6 and the actinides in period 7. Many actinides are radioactive, which signifies the limitations of nuclear stability at high atomic numbers. These elements further enrich the variety of chemical behaviors and provide insights into nuclear processes and radioactive decay.
The Significance of Periods: Unveiling Periodic Trends
The arrangement of elements into periods provides a powerful framework for understanding periodic trends. These trends are the systematic changes in elemental properties as we move across a period or down a group. Some key periodic trends directly influenced by period number include:
Atomic Radius: A Size Matters Perspective
As we move across a period from left to right, the atomic radius generally decreases. This is because the increasing nuclear charge attracts the electrons more strongly, pulling them closer to the nucleus, resulting in a smaller atom. However, going down a group, the atomic radius increases due to the addition of new electron shells.
Ionization Energy: The Energy to Remove an Electron
Ionization energy, the energy required to remove an electron from an atom, generally increases across a period. This is a direct consequence of the increasing nuclear charge. However, ionization energy decreases down a group as the outermost electrons are farther from the nucleus and shielded by inner electrons.
Electronegativity: The Tendency to Attract Electrons
Electronegativity, the tendency of an atom to attract electrons towards itself in a chemical bond, also generally increases across a period. This is linked to the increasing nuclear charge and decreasing atomic radius. Electronegativity decreases down a group as the outermost electrons are further from the nucleus and less tightly held.
Metallic Character: A Shift from Metals to Non-metals
The metallic character of elements generally decreases across a period. Metals are typically on the left side of the periodic table, characterized by their ability to readily lose electrons. As we move to the right, non-metals gain prominence, tending to gain electrons. This shift is reflected in their physical and chemical properties.
Deeper Insights into Periodicity: Subshells and Electron Configuration
The periodicity observed in the properties of elements arises from the way electrons fill the energy levels and subshells. This electron configuration dictates the chemical behavior of an element. Each period corresponds to the filling of a particular principal energy level (n=1, n=2, n=3, etc.), and the number of elements in a period reflects the number of orbitals available at that energy level.
Subshells and their Impact on Properties
The s, p, d, and f subshells have distinct shapes and energies. The filling order of these subshells, governed by the Aufbau principle and Hund's rule, determines the electronic structure of each element and its position in the periodic table. The unique characteristics of each subshell are responsible for the distinct properties observed within a period, especially within the transition metal and inner transition metal blocks.
Electron Configuration and Chemical Bonding
The electron configuration directly impacts an element's ability to form chemical bonds. Elements with partially filled outermost electron shells are more reactive, readily forming bonds to achieve a stable electron configuration. The number of electrons available for bonding, valence electrons, is also determined by the element's position in the periodic table, reflecting its period and group.
Beyond the Basics: Applications and Further Explorations
Understanding periods is essential for various chemical applications, including:
- Predicting chemical reactions: Knowledge of periodic trends allows chemists to predict the reactivity of elements and the types of bonds they are likely to form.
- Designing new materials: The periodic table is a fundamental tool in materials science, helping researchers select elements with specific properties to create new materials with desired characteristics.
- Understanding biological processes: Many essential biological molecules contain elements from various periods, and their properties are crucial for understanding their functions.
- Developing new technologies: The understanding of periodic trends fuels the innovation in fields like catalysis, energy storage, and electronics.
Conclusion: Periods – The Foundation of Chemical Understanding
The rows of the periodic table, known as periods, are far more than just horizontal lines. They represent a fundamental organizing principle reflecting the underlying electronic structure of atoms. By understanding the concept of periods, we unlock the key to understanding periodic trends and the systematic variations in the properties of elements. This knowledge forms the bedrock of chemical understanding and is essential for advancing scientific knowledge and technological innovation across diverse fields. The significance of periods extends beyond simple organization; it underpins our ability to predict chemical behaviors, design new materials, and comprehend complex natural processes. As we continue to explore the intricate world of chemistry, the importance of understanding periods remains paramount.
Latest Posts
Latest Posts
-
What Does The Spring Scale Measure
May 09, 2025
-
A Firms External Financing Need Is Met By
May 09, 2025
-
Find The Area Of Each Shaded Region Show Your Reasoning
May 09, 2025
-
Factor That Determines Where Aquatic Organisms Live
May 09, 2025
-
Is The Cheek Cell A Eukaryote Or Prokaryote
May 09, 2025
Related Post
Thank you for visiting our website which covers about What Are The Rows Of A Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.