What Are The Two Starting Materials For A Robinson Annulation
Muz Play
Mar 20, 2025 · 6 min read
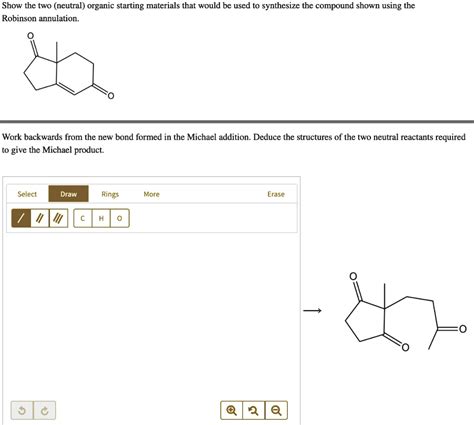
Table of Contents
What Are the Two Starting Materials for a Robinson Annulation?
The Robinson annulation is a powerful and elegant one-pot reaction in organic chemistry that forms six-membered rings. Its significance lies in its ability to efficiently construct complex cyclic structures from relatively simple starting materials, making it a cornerstone in the synthesis of numerous natural products and pharmaceuticals. Understanding the fundamental building blocks of this reaction—the two starting materials—is key to mastering its application and appreciating its synthetic utility. This comprehensive guide delves deep into the Robinson annulation, explaining the crucial roles of its two starting materials: a α,β-unsaturated ketone (or aldehyde) and a 1,3-dicarbonyl compound.
Understanding the Two Essential Components
The Robinson annulation is essentially a sequence of two reactions: a Michael addition followed by an intramolecular aldol condensation. This two-step process necessitates two specific types of reactants. Let's examine each one individually:
1. α,β-Unsaturated Ketone (or Aldehyde): The Michael Acceptor
The first crucial component is an α,β-unsaturated ketone (also known as an enone) or, less commonly, an α,β-unsaturated aldehyde. This molecule acts as the Michael acceptor in the initial step of the reaction. The key structural feature is the presence of a conjugated carbonyl group (C=O) and a carbon-carbon double bond (C=C). This conjugation creates a system where the electron-withdrawing carbonyl group polarizes the double bond, making the β-carbon electrophilic. This electrophilic β-carbon is the site of nucleophilic attack during the Michael addition.
Examples of α,β-unsaturated ketones include:
- Methyl vinyl ketone (MVK): This is a frequently used and readily available α,β-unsaturated ketone. Its simplicity and reactivity make it a popular choice for Robinson annulations.
- Cyclohexenone: This cyclic enone provides a pathway to synthesize fused ring systems, increasing the complexity and potential applications of the resulting product.
- Benzylideneacetone: The incorporation of an aromatic ring introduces further complexity and potential for diversification.
The choice of α,β-unsaturated ketone significantly influences the stereochemistry and overall structure of the final product. The substituents on the enone can affect the regioselectivity and stereoselectivity of both the Michael addition and the subsequent aldol condensation.
2. 1,3-Dicarbonyl Compound: The Michael Donor
The second essential starting material is a 1,3-dicarbonyl compound. This molecule functions as the Michael donor in the reaction. The crucial feature is the presence of two carbonyl groups separated by a single methylene group (-CH₂-). This arrangement allows for enolization – the formation of an enolate ion – which is the nucleophile that attacks the electrophilic β-carbon of the α,β-unsaturated ketone.
Examples of 1,3-dicarbonyl compounds include:
- Diethyl malonate: This diester is a versatile 1,3-dicarbonyl compound often used due to its accessibility and reactivity. The ester groups can be subsequently modified, offering opportunities for further functionalization of the final product.
- Ethyl acetoacetate: Similar to diethyl malonate, this compound is commonly employed and offers diverse possibilities for post-annulation transformations.
- Acetylacetone (pentane-2,4-dione): This symmetrical 1,3-diketone provides a simpler route to less functionalized Robinson annulation products.
The 1,3-dicarbonyl compound's pKa is relatively low due to the stabilization of the enolate anion by resonance. This makes the formation of the enolate ion readily achievable under basic conditions, crucial for the initiation of the Michael addition. The nature of the substituents on the 1,3-dicarbonyl compound also plays a role in influencing the reactivity and selectivity of the reaction.
The Mechanism: A Step-by-Step Breakdown
To fully appreciate the significance of the two starting materials, let's briefly examine the mechanism of the Robinson annulation:
Step 1: Michael Addition
Under basic conditions, the 1,3-dicarbonyl compound undergoes enolization. The resulting enolate ion acts as a nucleophile, attacking the electrophilic β-carbon of the α,β-unsaturated ketone. This 1,4-addition (Michael addition) forms a new carbon-carbon bond, creating an intermediate β-ketoester (or similar derivative depending on the 1,3-dicarbonyl compound used). This step is crucial, as it builds the carbon skeleton that will eventually form the six-membered ring. The reactivity of both the enolate and the enone are paramount for this step's success.
Step 2: Aldol Condensation
The intermediate from the Michael addition contains both a ketone and a carbonyl group (ester or ketone) suitably positioned for an intramolecular aldol condensation. Under the basic reaction conditions, the intermediate undergoes enolization again. This newly formed enolate ion performs an intramolecular aldol reaction, attacking the ketone carbonyl group. This creates a new carbon-carbon bond and forms a six-membered ring. This aldol product often undergoes dehydration to give an α,β-unsaturated ketone, the final product of the Robinson annulation.
Reaction Conditions and Optimization
The success of a Robinson annulation depends significantly on the careful control of reaction conditions. These typically include the choice of base, solvent, and temperature. Common bases used include potassium tert-butoxide (t-BuOK), sodium hydroxide (NaOH), and sodium ethoxide (NaOEt). The choice of base influences the rate and selectivity of both the Michael addition and the aldol condensation steps. Solvent selection is important to ensure proper solubility and reactivity.
Applications and Significance
The Robinson annulation holds immense value in organic synthesis due to its ability to efficiently construct complex cyclic structures in a single reaction. Its applications span numerous fields, including:
-
Natural Product Synthesis: The Robinson annulation has been extensively employed in the synthesis of various natural products, particularly steroids and terpenoids. Its capability to create fused six-membered rings makes it an indispensable tool in building the complex ring systems found in these molecules.
-
Pharmaceutical Chemistry: The reaction is vital in the development of various pharmaceuticals, allowing the creation of complex scaffolds that can be further modified to produce drug candidates. Its ability to efficiently assemble intricate molecules makes it a crucial step in medicinal chemistry.
-
Materials Science: The Robinson annulation can be employed to create building blocks for novel materials with specific properties. The ability to create precisely tailored ring systems allows for the design of materials with desired characteristics.
Variations and Modifications
While the classic Robinson annulation utilizes the specific starting materials described above, variations and modifications exist to expand its synthetic utility. These modifications often involve altering the reaction conditions, employing different catalysts, or using modified starting materials to achieve greater selectivity or access to more complex products. For instance, the use of different bases or solvents can favor certain reaction pathways, leading to different regio- or stereoisomers.
Conclusion
The Robinson annulation is a remarkably efficient and versatile reaction in organic chemistry. Its ability to efficiently create complex six-membered ring structures from relatively simple starting materials—a α,β-unsaturated ketone (or aldehyde) and a 1,3-dicarbonyl compound—has made it a cornerstone in numerous synthetic strategies. Understanding the fundamental roles of these two starting materials and the mechanism of the reaction is crucial for its successful application and for appreciating its impact on the broader field of organic synthesis and its applications in various scientific disciplines. The versatility and power of the Robinson annulation continue to inspire new synthetic approaches and remain a subject of ongoing research and optimization.
Latest Posts
Latest Posts
-
What Kind Of Change Forms A New Substance
Mar 21, 2025
-
1 2 Cos Alpha As A Product
Mar 21, 2025
-
Compound Interest And Simple Interest Worksheet
Mar 21, 2025
-
What Is A Sanction In Sociology
Mar 21, 2025
-
What Tool Is Used To Measure Mass
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Are The Two Starting Materials For A Robinson Annulation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
