What Do Fats Steroids And Waxes Have In Common
Muz Play
Apr 07, 2025 · 5 min read
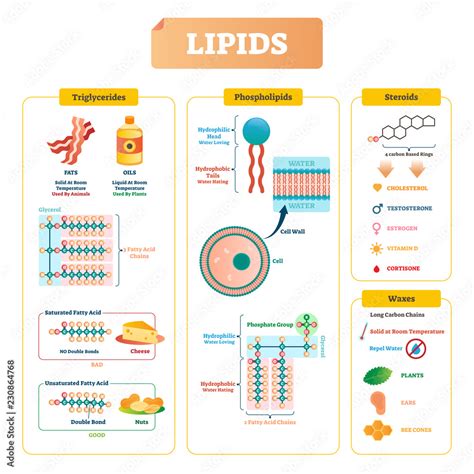
Table of Contents
What Do Fats, Steroids, and Waxes Have in Common? Exploring the World of Lipids
Lipids, a diverse group of naturally occurring organic compounds, are often misunderstood and lumped together as simply "fats." While fats are indeed a significant part of the lipid family, the group encompasses a much broader range of molecules, including steroids and waxes. Understanding what these seemingly disparate substances have in common requires delving into their fundamental chemical structure and properties. This article will explore the unifying characteristics of fats, steroids, and waxes, highlighting their similarities and key differences.
The Unifying Feature: Ester Bonds and Hydrocarbon Chains
The common thread that binds fats, steroids, and waxes together is their hydrophobic nature. This means they are insoluble in water. This property stems from their predominantly hydrocarbon makeup – long chains of carbon and hydrogen atoms. While they differ in their precise structures, all three groups contain significant portions of nonpolar hydrocarbon chains, leading to their insolubility in water.
Furthermore, many lipids, including fats and waxes, are characterized by the presence of ester bonds. An ester bond is formed through a dehydration reaction between a carboxylic acid and an alcohol. This bond is crucial in the formation of triglycerides (fats and oils) and many waxes.
Let's examine each group individually to understand their specific structures and how they relate to this unifying theme.
Fats (Triglycerides): The Energy Storage Champions
Fats, also known as triglycerides, are the most abundant type of lipid in our bodies and in many organisms. They are esters formed from the reaction of glycerol, a three-carbon alcohol, with three fatty acids. Fatty acids are long hydrocarbon chains with a carboxyl group (-COOH) at one end.
Structure of Fats:
- Glycerol backbone: The foundation of a fat molecule is glycerol, a small, three-carbon molecule with three hydroxyl (-OH) groups.
- Fatty acid chains: Each hydroxyl group on glycerol forms an ester bond with a fatty acid. The fatty acids can be saturated (containing only single bonds between carbon atoms), unsaturated (containing one or more double bonds), or polyunsaturated (containing multiple double bonds). The length and saturation of these fatty acid chains influence the physical properties of the fat, determining whether it's a solid (like butter) or a liquid (like olive oil) at room temperature.
Function of Fats:
The primary function of fats is energy storage. They are a highly efficient way for organisms to store energy, providing more than twice the energy per gram compared to carbohydrates. Additionally, fats serve as insulation, protecting vital organs and helping regulate body temperature. They also play a crucial role in the absorption of fat-soluble vitamins (A, D, E, and K).
Steroids: The Molecular Messengers
Steroids are a distinct group of lipids characterized by their unique four-ring structure. This structure, consisting of three cyclohexane rings and one cyclopentane ring, forms the core of all steroid molecules. Unlike fats and waxes, steroids do not typically contain fatty acids.
Structure of Steroids:
- Four-ring core: The common feature is the fused ring system, often referred to as the steroid nucleus.
- Variable side chains: The specific arrangement of functional groups and side chains attached to this core determines the unique properties and functions of different steroids.
Function of Steroids:
Steroids have diverse roles in biological systems. Perhaps the most well-known steroid is cholesterol, a crucial component of cell membranes that maintains membrane fluidity. Steroid hormones, such as testosterone, estrogen, and cortisol, act as messengers, regulating a wide range of physiological processes, including growth, development, metabolism, and stress response.
Waxes: The Protective Coatings
Waxes are esters formed from a long-chain fatty acid and a long-chain alcohol. Unlike triglycerides, they don't contain glycerol. They are typically solid at room temperature and possess a high melting point.
Structure of Waxes:
- Long-chain fatty acid: One component is a long-chain carboxylic acid (often containing 14-36 carbon atoms).
- Long-chain alcohol: The other component is a long-chain alcohol (also with a long hydrocarbon chain). These chains are usually saturated, contributing to the wax's solidity.
Function of Waxes:
Waxes primarily serve as protective coatings in living organisms. They are found in plant cuticles, providing waterproofing and protection against desiccation. Animal waxes, such as beeswax and lanolin, serve similar protective roles. Their hydrophobic nature makes them excellent barriers against water and other environmental factors.
Connecting the Dots: Commonalities and Differences Summarized
| Feature | Fats (Triglycerides) | Steroids | Waxes |
|---|---|---|---|
| Basic Structure | Glycerol + 3 Fatty Acids | Four-ring fused structure | Long-chain fatty acid + long-chain alcohol |
| Ester Bonds | Present | Typically Absent | Present |
| Hydrocarbon Chains | Abundant | Present (in side chains) | Abundant |
| Hydrophobicity | High | High | High |
| Primary Function | Energy storage, insulation | Hormone regulation, membrane structure | Protection, waterproofing |
Beyond the Basics: Exploring Further Connections
While the primary structural differences are clear, there are subtle yet significant interconnections between these lipid classes. For example, cholesterol, a crucial steroid, is essential for the synthesis of steroid hormones and bile acids. Furthermore, some fatty acids are precursors to the synthesis of other lipids, including certain steroids. The metabolic pathways involving these molecules are complex and interconnected, demonstrating the intricate interplay between different lipid classes.
Implications and Applications
Understanding the properties and functions of fats, steroids, and waxes has significant implications in various fields. In the food industry, the composition and properties of fats are crucial determinants of food texture, flavor, and shelf life. In medicine, steroids are widely used as drugs to treat various conditions, while the understanding of lipid metabolism is essential in managing cardiovascular diseases. In cosmetics and industry, waxes find numerous applications due to their water-repellent and protective properties.
Conclusion: A Unified Family of Hydrophobic Molecules
Fats, steroids, and waxes, despite their structural diversity, belong to the unified family of lipids. Their commonality lies in their hydrophobic nature stemming from their abundant hydrocarbon chains. While fats excel as energy stores, steroids serve as molecular messengers, and waxes provide protective coatings, all three share the fundamental characteristic of insolubility in water and play essential roles in biological systems and diverse human applications. Further research into the intricate connections and functions of lipids promises to reveal even more fascinating insights into the biochemistry of life.
Latest Posts
Latest Posts
-
What Are The Most Reactive Alkali Metals
Apr 09, 2025
-
What Is The Purpose Of Boiling Stones
Apr 09, 2025
-
How To Identify Bacteria On Agar Plates
Apr 09, 2025
-
How To Find Which Isotope Is More Abundant
Apr 09, 2025
-
L Is Known As The Quantum Number
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about What Do Fats Steroids And Waxes Have In Common . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.