What Does A Negative Delta G Mean
Muz Play
Mar 26, 2025 · 6 min read
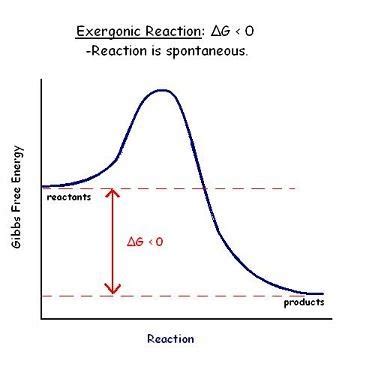
Table of Contents
What Does a Negative Delta G Mean? Understanding Gibbs Free Energy
The concept of Gibbs Free Energy and its change (ΔG) is fundamental to understanding the spontaneity of chemical reactions and physical processes. A negative ΔG signifies a spontaneous process, but what does this really mean? This article delves deep into the meaning of a negative ΔG, exploring its implications in various contexts, including thermodynamics, biochemistry, and everyday life. We'll break down the intricacies of Gibbs Free Energy and explain why a negative value holds such significance.
Understanding Gibbs Free Energy (G)
Before we delve into the meaning of a negative ΔG, let's first establish a solid understanding of Gibbs Free Energy itself. Gibbs Free Energy (G) is a thermodynamic potential that measures the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressure. It's a crucial concept because it combines enthalpy (H), a measure of the system's total energy, and entropy (S), a measure of the system's disorder, to predict the spontaneity of a process.
The relationship is defined by the equation:
G = H - TS
Where:
- G is the Gibbs Free Energy
- H is the enthalpy (heat content)
- T is the absolute temperature (in Kelvin)
- S is the entropy (disorder)
This equation tells us that the Gibbs Free Energy is influenced by both the system's energy content and its disorder. A decrease in enthalpy (exothermic reaction, releasing heat) or an increase in entropy (increase in disorder) will favor a decrease in Gibbs Free Energy.
The Significance of Delta G (ΔG)
Delta G (ΔG) represents the change in Gibbs Free Energy during a process. It's calculated as the difference between the Gibbs Free Energy of the products and the Gibbs Free Energy of the reactants:
ΔG = G<sub>products</sub> - G<sub>reactants</sub>
The sign and magnitude of ΔG are crucial in determining whether a process will occur spontaneously under specific conditions (constant temperature and pressure).
What Does a Negative ΔG Mean?
A negative ΔG indicates that a process is spontaneous under the given conditions. This means the process will occur naturally without any external input of energy. The reaction will proceed in the forward direction towards equilibrium. The magnitude of the negative ΔG indicates the driving force behind the spontaneity; a larger negative value suggests a more strongly favored reaction.
Spontaneity: A Deeper Dive
It's crucial to understand that spontaneity doesn't necessarily mean a reaction will occur quickly. Spontaneity refers to the thermodynamic favorability of a reaction, not its kinetics (rate). A reaction can be spontaneous (negative ΔG) but proceed very slowly due to high activation energy. This is where catalysts play a vital role, lowering the activation energy to accelerate the reaction without affecting the overall ΔG.
Factors Affecting ΔG
Several factors influence the value of ΔG, making it a dynamic indicator of a reaction's tendency to proceed:
1. Enthalpy (ΔH)
- Exothermic reactions (ΔH < 0): These reactions release heat, contributing to a negative ΔG. The release of heat increases the stability of the system.
- Endothermic reactions (ΔH > 0): These reactions absorb heat, making a negative ΔG less likely. The absorption of heat decreases the stability of the system. A spontaneous endothermic reaction must be driven by a significant increase in entropy.
2. Entropy (ΔS)
- Increase in entropy (ΔS > 0): An increase in disorder (more randomness) favors a negative ΔG. Systems tend towards higher entropy.
- Decrease in entropy (ΔS < 0): A decrease in disorder (more order) works against a negative ΔG. Systems resist decreases in entropy.
3. Temperature (T)
Temperature plays a crucial role in the interplay between enthalpy and entropy. The term "-TΔS" in the Gibbs Free Energy equation shows that the effect of entropy on spontaneity is temperature-dependent.
- High temperatures: At high temperatures, the "-TΔS" term becomes more significant. Therefore, even if an endothermic reaction (positive ΔH) has a positive ΔS, it could become spontaneous at a sufficiently high temperature.
- Low temperatures: At low temperatures, the influence of the "-TΔS" term is reduced. Spontaneous reactions at low temperatures are more likely to be exothermic (negative ΔH) with a small or positive ΔS.
The Relationship Between ΔG, Equilibrium, and Equilibrium Constant (K)
The change in Gibbs Free Energy is also directly related to the equilibrium constant (K) of a reversible reaction at a given temperature:
ΔG° = -RTlnK
Where:
- ΔG° is the standard Gibbs Free Energy change (at standard conditions, usually 298K and 1 atm)
- R is the ideal gas constant
- T is the temperature in Kelvin
- K is the equilibrium constant
This equation highlights the connection between thermodynamics and kinetics. A large equilibrium constant (K >> 1) indicates that the products are strongly favored at equilibrium, resulting in a large negative ΔG°. Conversely, a small equilibrium constant (K << 1) implies that the reactants are favored, leading to a positive ΔG°. A K value of 1 indicates that the reaction is at equilibrium, with ΔG° = 0.
Applications of Negative ΔG
The concept of a negative ΔG has wide-ranging applications across various scientific fields:
1. Biochemistry: Metabolic Processes
Many metabolic processes in living organisms rely on negative ΔG to drive reactions forward. For example, the breakdown of glucose during cellular respiration is a spontaneous process with a highly negative ΔG, providing the energy necessary for life's functions. Coupled reactions, where a spontaneous reaction (negative ΔG) drives a non-spontaneous one, are crucial in biochemical pathways.
2. Chemistry: Reaction Spontaneity
In chemistry, understanding ΔG is vital for predicting whether a reaction will proceed spontaneously. Chemists use this information to design experiments, optimize reaction conditions, and develop new chemical processes.
3. Environmental Science: Predicting Natural Processes
Negative ΔG helps predict the spontaneity of natural processes like the dissolution of minerals, the flow of water downhill, and the diffusion of gases. Understanding these spontaneous processes is crucial for modeling environmental systems and predicting their behavior.
4. Materials Science: Material Stability
The stability of materials can also be assessed using Gibbs Free Energy. A negative ΔG for a transformation indicates that the transformation is thermodynamically favored, providing insight into material behavior and degradation.
Limitations of ΔG
While ΔG is a powerful tool, it has some limitations:
- It does not provide information about the rate of reaction. A reaction can be thermodynamically favorable (negative ΔG) but kinetically slow due to a high activation energy barrier.
- It only applies to systems at constant temperature and pressure. ΔG is not applicable to systems where temperature or pressure change significantly during the process.
- It doesn't consider the presence of catalysts. Catalysts speed up reactions without affecting the overall ΔG.
Conclusion: The Power of a Negative ΔG
A negative ΔG signifies a spontaneous process, a fundamental concept in thermodynamics with far-reaching implications. Understanding this concept allows scientists and engineers to predict the behavior of systems, design efficient processes, and develop new technologies. Whether in biochemistry, chemistry, environmental science, or materials science, the ability to predict spontaneity through the analysis of Gibbs Free Energy is invaluable for understanding and manipulating the world around us. The interplay of enthalpy, entropy, and temperature ultimately determines the fate of a process, making the interpretation of ΔG a critical skill in multiple scientific disciplines. Remember, however, that while a negative ΔG indicates spontaneity, it doesn't dictate the reaction rate, highlighting the importance of considering both thermodynamics and kinetics for a complete understanding.
Latest Posts
Latest Posts
-
Mannitol Salt Agar Is Selective For Which Bacterial Genus
Mar 29, 2025
-
How To Do Statistical Data Transformations In Excel
Mar 29, 2025
-
Why Are Hydrogen Bonds Important For Life
Mar 29, 2025
-
Amount Of Lime To Neutralie 9 Lbs Of Solfuric Acid
Mar 29, 2025
-
Why Are Base Pairing Rules Important
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Does A Negative Delta G Mean . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
