What Does High Km Mean Enzyme
Muz Play
Mar 17, 2025 · 5 min read
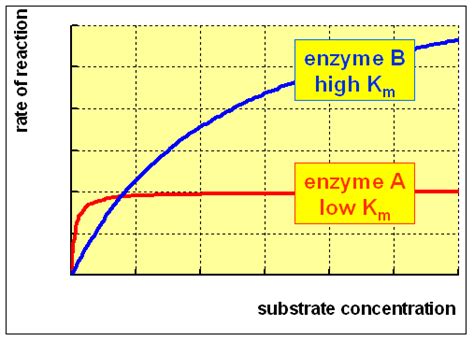
Table of Contents
What Does High Km Mean for an Enzyme? Understanding Michaelis-Menten Kinetics
Enzymes are biological catalysts that accelerate the rate of biochemical reactions within cells. Understanding their kinetics is crucial to comprehending cellular processes and developing pharmaceuticals. A key parameter in enzyme kinetics is the Michaelis constant, or Km. This article delves into the meaning of a high Km value, its implications, and its broader significance in biochemistry and medicine.
The Michaelis-Menten Equation: A Foundation for Understanding Km
The Michaelis-Menten equation is a cornerstone of enzyme kinetics. It describes the relationship between the initial reaction velocity (V₀) of an enzyme-catalyzed reaction and the concentration of the substrate ([S]). The equation is expressed as:
V₀ = (Vmax[S]) / (Km + [S])
Where:
- V₀: Initial reaction velocity
- Vmax: Maximum reaction velocity (when the enzyme is saturated with substrate)
- [S]: Substrate concentration
- Km: Michaelis constant
The Michaelis constant (Km) is defined as the substrate concentration at which the reaction velocity is half of the maximum velocity (Vmax/2). It's a crucial indicator of an enzyme's affinity for its substrate.
What Does a High Km Value Indicate?
A high Km value signifies a low affinity of the enzyme for its substrate. This means that a higher concentration of substrate is required to achieve half of the maximum reaction velocity. In simpler terms, the enzyme needs more substrate molecules to be "present" to work effectively.
Think of it like this: imagine an enzyme trying to bind to a substrate. If the Km is high, it's like the enzyme is somewhat clumsy or doesn't "recognize" the substrate easily. It needs many substrate molecules to bump into it before a successful binding event occurs, leading to a slower reaction at lower substrate concentrations.
Conversely, a low Km value indicates a high affinity, meaning the enzyme binds to its substrate readily even at low substrate concentrations, leading to a faster reaction.
Practical Implications of a High Km
A high Km value has several practical implications:
-
Lower catalytic efficiency at low substrate concentrations: At low substrate concentrations, enzymes with high Km values will exhibit significantly lower catalytic activity compared to enzymes with low Km values. This is because they require a much higher concentration of substrate to reach their half-maximal velocity.
-
Greater substrate concentration required for optimal activity: Achieving optimal enzyme activity requires a substantially higher substrate concentration for high-Km enzymes. This can have significant consequences in biological systems, where substrate availability may be limited.
-
Potential for competitive inhibition: High Km values might suggest a higher susceptibility to competitive inhibitors. These inhibitors compete with the substrate for binding to the enzyme's active site. A high Km means the enzyme's affinity for its substrate is already relatively weak, making it more vulnerable to displacement by a competitive inhibitor.
-
Implications for drug design: In drug design, understanding the Km value of target enzymes is crucial. If the target enzyme has a high Km, designing drugs that can act as competitive inhibitors to reduce its activity might be a more viable therapeutic strategy.
Factors Influencing Km Values
Several factors can influence the Km value of an enzyme:
-
Enzyme structure: The three-dimensional structure of the enzyme, including the active site, plays a critical role in determining its affinity for the substrate. Mutations or alterations in the enzyme's structure can significantly alter the Km value.
-
pH and temperature: Changes in pH and temperature can affect the enzyme's conformation and thus its ability to bind to the substrate, altering the Km value. Optimal pH and temperature values often result in the lowest Km value, reflecting the highest affinity.
-
Presence of allosteric effectors: Allosteric effectors are molecules that bind to the enzyme at a site other than the active site, influencing its activity. They can either increase or decrease the enzyme's affinity for the substrate, leading to changes in the Km value. Allosteric activators typically decrease Km, while inhibitors increase it.
-
Substrate structure: The structure and properties of the substrate itself influence its interaction with the enzyme. Variations in substrate structure can significantly affect the Km value, sometimes leading to significant differences in the enzyme's affinity.
High Km and its Significance in Different Biological Contexts
The significance of a high Km value varies greatly depending on the biological context.
Metabolic pathways: In metabolic pathways, enzymes with high Km values often play regulatory roles. Their activity is highly sensitive to changes in substrate concentration, allowing for fine-tuning of metabolic flux. This is particularly crucial in pathways where substrate availability fluctuates significantly.
Drug metabolism: Enzymes involved in drug metabolism frequently exhibit a range of Km values. Understanding these Km values is vital in predicting drug clearance, duration of action, and potential drug interactions. A high Km value for a metabolic enzyme might indicate a slower drug metabolism rate.
Disease states: Changes in enzyme Km values can be indicative of certain disease states. For example, mutations in enzymes leading to significantly altered Km values are frequently implicated in various genetic disorders. These changes can manifest as reduced catalytic efficiency or altered regulation of metabolic pathways, contributing to disease pathology.
Experimental Determination of Km
The Km value of an enzyme is typically determined experimentally using methods like:
-
Direct measurement: Involves directly measuring the reaction velocity at various substrate concentrations and plotting the data on a Michaelis-Menten graph or a Lineweaver-Burk plot (a double reciprocal plot of the Michaelis-Menten equation). The Km is determined from the graph.
-
Indirect estimation: Involves using indirect techniques, such as competitive inhibition studies, which can provide information on the Km value indirectly.
Precise determination of Km requires careful experimental design and analysis to account for potential errors and biases.
Conclusion: A High Km is Context-Dependent
A high Km value for an enzyme signifies a low affinity for its substrate. This has far-reaching implications for the enzyme's activity, regulation, and role in various biological processes. The significance of a high Km, however, is highly context-dependent, varying across different biological systems and metabolic pathways. Understanding the Km value and its factors are crucial in comprehending enzyme function and designing effective therapeutic strategies targeting enzymatic activity. Further research is constantly refining our understanding of enzyme kinetics, providing valuable insights into the complexity and regulation of biological systems. The Michaelis-Menten equation and the Km value remain foundational tools in this endeavor.
Latest Posts
Latest Posts
-
Which Body Cavity Protects The Spinal Column
Mar 17, 2025
-
Sampling Distribution Of The Sample Mean Calculator
Mar 17, 2025
-
Expected Value Of A Discrete Random Variable
Mar 17, 2025
-
Calculate Generation Time From Growth Curve
Mar 17, 2025
-
Factors That Affect Elasticity Of Supply
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Does High Km Mean Enzyme . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
