What Does Yield Mean In Chemistry
Muz Play
Mar 23, 2025 · 5 min read
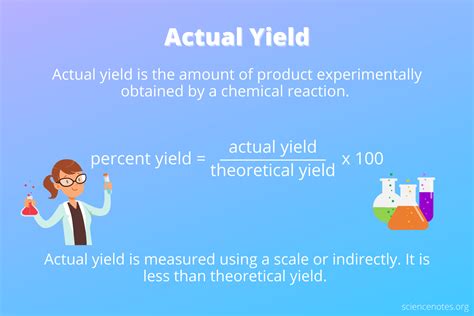
Table of Contents
What Does Yield Mean in Chemistry? A Comprehensive Guide
Yield, in chemistry, refers to the amount of product obtained from a chemical reaction. Understanding yield is crucial for optimizing chemical processes, predicting reaction outcomes, and evaluating the efficiency of a synthetic route. This comprehensive guide delves into the intricacies of yield, exploring its different types, calculations, factors influencing it, and its significance in various chemical applications.
Types of Yield in Chemistry
Several types of yield are used in chemistry, each providing different insights into the efficiency of a reaction:
1. Actual Yield
This refers to the actual amount of product obtained from a chemical reaction. It's the mass of the purified product you physically isolate at the end of your experiment. It's a practical, experimentally determined value, often expressed in grams or moles. The actual yield is always less than or equal to the theoretical yield due to unavoidable losses during the process.
2. Theoretical Yield
The theoretical yield represents the maximum amount of product that could be obtained from a reaction if it proceeds completely according to the stoichiometry (the mole ratio of reactants and products in a balanced chemical equation). It's a calculated value, based on the complete conversion of the limiting reactant. It's an idealized scenario, assuming perfect reaction conditions and 100% efficiency.
3. Percent Yield
This is the most commonly used measure of reaction efficiency. The percent yield compares the actual yield to the theoretical yield, expressing the efficiency of the reaction as a percentage.
Percent Yield = (Actual Yield / Theoretical Yield) x 100%
A high percent yield (close to 100%) indicates a highly efficient reaction, while a low percent yield suggests significant losses or incomplete reaction. Factors influencing the percent yield are discussed in detail below.
Calculating Yield: A Step-by-Step Guide
Let's illustrate yield calculations with a simple example:
Reaction: 2H₂ + O₂ → 2H₂O
Suppose we react 2 grams of Hydrogen (H₂) with excess Oxygen (O₂). We obtain 15 grams of water (H₂O) experimentally.
1. Calculate the Moles of Reactant:
- Molar mass of H₂ = 2 g/mol
- Moles of H₂ = (mass of H₂) / (molar mass of H₂) = 2 g / 2 g/mol = 1 mol
2. Calculate the Theoretical Moles of Product:
According to the stoichiometry, 2 moles of H₂ produce 2 moles of H₂O. Therefore, 1 mol of H₂ will produce 1 mol of H₂O.
3. Calculate the Theoretical Yield (in grams):
- Molar mass of H₂O = 18 g/mol
- Theoretical yield of H₂O = (theoretical moles of H₂O) x (molar mass of H₂O) = 1 mol x 18 g/mol = 18 g
4. Calculate the Percent Yield:
- Actual yield of H₂O = 15 g
- Percent yield = (15 g / 18 g) x 100% = 83.33%
This example shows that the reaction is 83.33% efficient, meaning that some water was lost during the reaction or purification process.
Factors Affecting Yield
Several factors can significantly influence the actual yield and consequently the percent yield of a chemical reaction. These include:
1. Reaction Conditions:
- Temperature: Temperature affects reaction rates. Optimal temperatures maximize product formation. Too high a temperature might lead to side reactions or product degradation.
- Pressure: Especially important for gaseous reactions, pressure can influence the equilibrium position and reaction rate.
- Concentration of Reactants: Higher concentrations of reactants generally lead to faster reaction rates and potentially higher yields, but this is not always the case and depends on the reaction kinetics.
- Solvent: The choice of solvent can affect the solubility of reactants and products, influencing the reaction rate and yield.
- Catalyst: Catalysts accelerate reactions without being consumed. They can significantly increase yields by lowering the activation energy.
2. Side Reactions:
Unwanted side reactions compete with the main reaction, consuming reactants and producing undesired byproducts. This reduces the amount of the desired product, leading to a lower yield.
3. Incomplete Reactions:
Reactions often do not go to completion. Equilibrium may be established before all the limiting reactant is consumed.
4. Purification Losses:
During purification processes (e.g., recrystallization, distillation, chromatography), some product is inevitably lost. This is a significant factor that reduces the actual yield. Improper purification techniques can severely impact the yield.
5. Experimental Errors:
Human errors, such as inaccurate measurements, incorrect procedure, or incomplete collection of product, can significantly affect the actual yield.
6. Purity of Reactants:
Impurities in the starting materials can affect the reaction and reduce the yield. The presence of contaminants may interfere with the reaction mechanism or even lead to side reactions.
Importance of Yield in Chemical Industries
Yield is a critical parameter in various chemical industries:
- Pharmaceutical Industry: High yields are crucial for cost-effectiveness in drug synthesis. Low yields mean higher production costs and potentially limit the availability of essential drugs.
- Polymer Industry: Efficient polymerization processes require high yields to maximize polymer production and minimize waste.
- Agricultural Chemistry: In fertilizer production, high yields are essential for maximizing the amount of active ingredient, increasing crop yields and reducing the environmental impact.
- Petrochemical Industry: Optimizing reaction yields in refining processes is vital for maximizing the production of valuable chemicals from crude oil.
Optimizing Yield: Strategies and Techniques
Improving reaction yields often requires careful optimization of reaction conditions and the use of advanced techniques:
- Careful Control of Reaction Parameters: Precise control of temperature, pressure, reactant concentrations, and reaction time is crucial for maximizing the yield.
- Use of Catalysts: Employing efficient catalysts can significantly improve yields by increasing reaction rates and selectivity.
- Improving Purification Techniques: Implementing efficient and optimized purification techniques minimizes product loss during isolation and purification.
- Reaction Monitoring and Optimization: Techniques like in-situ monitoring (e.g., using NMR, IR) can provide valuable information for optimizing reaction conditions in real-time.
- Process Development and Scale-up: Moving from laboratory-scale reactions to industrial-scale processes often requires careful optimization of reaction conditions and design of efficient reactors.
Conclusion
Yield is a fundamental concept in chemistry, providing a quantitative measure of the efficiency of a chemical reaction. Understanding the different types of yield, their calculations, and the factors that influence them is crucial for chemists and engineers across various industries. Optimizing reaction yields is critical for cost-effectiveness, resource efficiency, and the sustainability of chemical processes. By meticulously controlling reaction conditions, using appropriate catalysts, and employing advanced purification techniques, chemists can strive for high yields, maximizing product output and minimizing waste. The pursuit of high yields remains a central focus in the continuous improvement and advancement of chemical synthesis and industrial processes.
Latest Posts
Latest Posts
-
The Calvin Cycle Occurs In The
Mar 24, 2025
-
Broadband Is Usually Measured In
Mar 24, 2025
-
Comparison Of Skeletal Cardiac And Smooth Muscle
Mar 24, 2025
-
How To Find Vertices Of Ellipse
Mar 24, 2025
-
Venn Diagram All S Are P
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about What Does Yield Mean In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
