What Elemsnt Are Most Likey To Turn Into Anions Why
Muz Play
Mar 15, 2025 · 5 min read
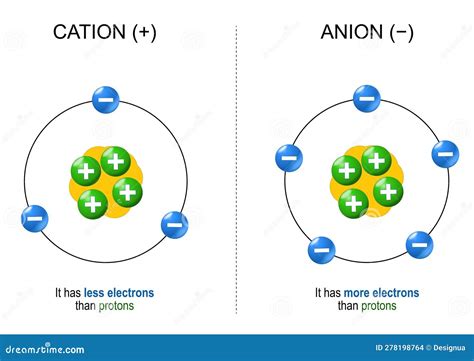
Table of Contents
What Elements Are Most Likely to Turn into Anions? Why?
Understanding the formation of anions – negatively charged ions – is fundamental to chemistry. This article delves deep into the factors that determine an element's propensity to gain electrons and become an anion. We'll explore electronegativity, ionization energy, and electron affinity, linking these concepts to the periodic table's structure and providing concrete examples.
Understanding Anion Formation
Anions are formed when an atom gains one or more electrons. This gain of negatively charged electrons results in a net negative charge on the atom, transforming it into an anion. The driving force behind this electron gain lies in the atom's electronic structure and its inherent tendency to achieve a stable, lower-energy state. This stable state is often achieved by filling its outermost electron shell (valence shell) to a full octet (eight electrons) – a configuration similar to the noble gases, which are exceptionally stable.
Key Factors Governing Anion Formation
Several key properties influence an element's likelihood of forming anions:
1. Electronegativity: The Electron-Hogging Ability
Electronegativity is a crucial factor. It measures an atom's ability to attract electrons towards itself within a chemical bond. Elements with high electronegativity are more likely to attract electrons and form anions when bonding with less electronegative elements. The Pauling scale quantifies electronegativity, with fluorine (F) possessing the highest value (4.0). Elements with high electronegativity are found in the upper right corner of the periodic table (excluding noble gases).
Examples of High Electronegativity Elements:
- Fluorine (F): Forms fluoride anions (F⁻) readily.
- Oxygen (O): Forms oxide anions (O²⁻) commonly.
- Chlorine (Cl): Forms chloride anions (Cl⁻) extensively.
- Nitrogen (N): Forms nitride anions (N³⁻) though less readily than halogens.
2. Ionization Energy: The Energy Cost of Losing an Electron
Ionization energy represents the energy required to remove an electron from a neutral atom. While not directly related to anion formation in the same way as electronegativity, it provides valuable context. Elements with low ionization energies are less likely to lose electrons, making it relatively easier for them to gain electrons and form anions. This is because losing an electron requires significant energy, and if an atom isn't readily losing them, gaining electrons is more likely a path to stability. High ionization energies, conversely, suggest the element is holding tightly to its electrons and thus less likely to form anions.
3. Electron Affinity: The Energy Change Upon Electron Gain
Electron affinity describes the energy change that occurs when a neutral atom gains an electron. A high positive electron affinity indicates that the atom releases energy upon gaining an electron, making anion formation energetically favorable. A negative electron affinity implies that energy is required to add an electron, making anion formation less likely. However, it is important to note that electron affinity values can vary with the addition of multiple electrons.
The Periodic Table and Anion Formation: A Trend Analysis
The periodic table organizes elements based on their properties, providing a clear visual representation of anion formation tendencies:
Group 17 (Halogens): The Anion Champions
Halogens (fluorine, chlorine, bromine, iodine, astatine) are the most prominent anion-formers. Their high electronegativity and relatively high electron affinities make gaining an electron highly favorable. They readily achieve a stable octet by gaining a single electron, forming halide anions (F⁻, Cl⁻, Br⁻, I⁻, At⁻).
Group 16 (Chalcogens): Oxygen's Reign
Oxygen and other chalcogens (sulfur, selenium, tellurium, polonium) also have a strong tendency to form anions. While not as pronounced as halogens, their high electronegativity leads to them typically gaining two electrons to achieve a stable octet, forming anions like oxide (O²⁻), sulfide (S²⁻), selenide (Se²⁻), etc.
Group 15 (Pnictogens): A More Nuanced Picture
Nitrogen and the other pnictogens exhibit a more complex behavior. While they can form anions (like nitride, N³⁻), their electronegativity is lower than halogens and chalcogens. Therefore, anion formation is less spontaneous and often requires specific reaction conditions.
Group 14 (Tetrels): Carbon's Anionic Exception
Carbon, typically forming covalent bonds, can form carbide anions (C⁴⁻) under certain circumstances, specifically with highly electropositive metals. However, this is less common than anion formation in groups 15-17.
Metals: Anionic Formation is Uncommon
Metals generally have low electronegativities and low electron affinities. They tend to lose electrons to form cations (positively charged ions) rather than gaining electrons to form anions. Exceptions exist, notably some transition metals forming complex anions under specific chemical environments.
Beyond the Basics: Factors Influencing Anion Stability
Ionic Radius: Size Matters
Larger ionic radii generally lead to greater anion stability. This is due to the increased distance between the nucleus and the added electrons, reducing the electrostatic attraction and lessening the repulsion among the electrons already present.
Charge Density: Balancing Act
The charge density (charge per unit volume) influences anion stability. A higher charge density indicates a higher concentration of negative charge in a smaller space, leading to increased electron-electron repulsion, which destabilizes the anion.
Covalent Character: A Blurred Line
In some compounds, the bond between the anion-forming element and another element might exhibit a significant covalent character, blurring the line between purely ionic and covalent bonding. The degree of covalent character depends on the electronegativity difference between the elements involved.
Conclusion: A Spectrum of Anion Formation
Anion formation isn't a binary phenomenon; it exists on a spectrum. While some elements, such as halogens, demonstrate a very strong tendency to form anions, others show a weaker or context-dependent propensity. Understanding electronegativity, electron affinity, and ionization energy, coupled with insights from the periodic table, provides a robust framework for predicting an element's likelihood of forming anions. Moreover, the influence of factors such as ionic radius and covalent character adds complexity and nuance to the phenomenon, highlighting the intricate interplay of forces governing chemical bonding and ion formation. This comprehensive understanding is essential for mastering fundamental chemistry and venturing into more advanced chemical concepts.
Latest Posts
Latest Posts
-
The Most Prominent Organelle In A Eukaryotic Cell Is The
May 09, 2025
-
Density Of Soil In Kg M3
May 09, 2025
-
Are Nuclei Visible In Cyanobacterial Cells
May 09, 2025
-
Is The Mean Greater Than The Median In Right Skewed
May 09, 2025
-
Compare And Contrast Spermatogenesis And Oogenesis In Human Cells
May 09, 2025
Related Post
Thank you for visiting our website which covers about What Elemsnt Are Most Likey To Turn Into Anions Why . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.