What Happens When An Atom Gains Or Loses An Electron
Muz Play
Apr 01, 2025 · 6 min read
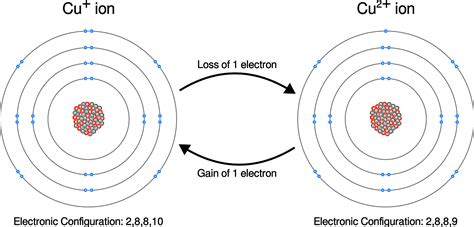
Table of Contents
What Happens When an Atom Gains or Loses an Electron?
The seemingly simple act of an atom gaining or losing an electron has profound consequences, shaping the properties of matter and driving countless chemical and physical processes in the universe. Understanding this fundamental interaction is key to grasping the intricacies of chemistry, physics, and materials science. This article delves deep into the implications of electron transfer, exploring the resulting charged particles, their behavior, and the broader impact on the macroscopic world.
The Basics: Electrons, Protons, and Neutrons
Before examining the consequences of electron gain or loss, let's revisit the fundamental components of an atom:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element (e.g., one proton for hydrogen, six for carbon).
- Neutrons: Neutral particles also residing in the nucleus. They contribute to the atom's mass but not its charge. Isotopes of an element differ in the number of neutrons.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. They are significantly lighter than protons and neutrons. The number of electrons typically equals the number of protons in a neutral atom.
Ionization: The Birth of Ions
When an atom gains or loses electrons, it no longer possesses a neutral charge. This process is called ionization, and the resulting charged particle is known as an ion.
Cation Formation: Electron Loss
When an atom loses one or more electrons, it becomes positively charged because the number of protons now exceeds the number of electrons. This positively charged ion is called a cation. The formation of cations is influenced by several factors, including:
- Electro negativity: Atoms with low electronegativity, meaning they have a weaker hold on their valence electrons, are more likely to lose electrons and form cations. These are generally found on the left side of the periodic table (alkali and alkaline earth metals).
- Ionization energy: The energy required to remove an electron from an atom. Atoms with low ionization energies readily lose electrons.
- Stability: Atoms tend to lose electrons to achieve a stable electron configuration, often a full outer shell (octet rule).
Anion Formation: Electron Gain
Conversely, when an atom gains one or more electrons, it becomes negatively charged because the number of electrons surpasses the number of protons. This negatively charged ion is called an anion. Anion formation is driven by:
- Electronegativity: Atoms with high electronegativity, meaning they strongly attract electrons, readily gain electrons to form anions. These are generally found on the right side of the periodic table (halogens and chalcogens).
- Electron affinity: The energy change that occurs when an atom gains an electron. Atoms with high electron affinities readily accept electrons.
- Stability: Similar to cations, atoms gain electrons to achieve a stable electron configuration, often filling their outer shell.
The Consequences of Ion Formation: A Ripple Effect
The formation of ions has a cascading effect on the physical and chemical properties of the involved atoms and subsequently the materials they form:
1. Changes in Chemical Reactivity:
Ions exhibit drastically different chemical properties compared to their neutral atom counterparts. Their charge significantly alters their interactions with other atoms and molecules. Cations are often electropositive, readily reacting with electronegative anions to form ionic compounds. Anions, being electronegative, attract positively charged species.
2. Altered Physical Properties:
Ionic compounds, formed by the electrostatic attraction between cations and anions, display distinct physical properties compared to their constituent elements. These properties include:
- High melting and boiling points: The strong electrostatic forces between ions require significant energy to overcome.
- Crystalline structure: Ions arrange themselves in a regular, repeating pattern in a crystal lattice.
- Solubility in polar solvents: Ionic compounds readily dissolve in polar solvents like water due to the interaction between the ions and the solvent molecules.
- Electrical conductivity: When molten or dissolved in a polar solvent, ionic compounds conduct electricity because the ions are free to move and carry charge.
3. Impact on Biological Systems:
Ion formation is crucial for numerous biological processes. For example:
- Nerve impulse transmission: The movement of sodium (Na⁺) and potassium (K⁺) ions across nerve cell membranes is essential for transmitting nerve impulses.
- Muscle contraction: Calcium (Ca²⁺) ions play a vital role in muscle contraction.
- Enzyme function: Many enzymes require specific ions as cofactors to function properly.
- Maintaining osmotic balance: Ions contribute to maintaining the proper osmotic balance within cells and tissues.
4. Formation of Ionic Compounds:
The electrostatic attraction between oppositely charged ions leads to the formation of ionic compounds. These compounds are characterized by strong ionic bonds, resulting in the properties mentioned above. Examples include sodium chloride (NaCl), table salt, and calcium oxide (CaO), quicklime.
5. Redox Reactions:
The transfer of electrons during ionization is a fundamental aspect of redox (reduction-oxidation) reactions. Redox reactions involve the simultaneous oxidation (loss of electrons) of one species and the reduction (gain of electrons) of another. These reactions are essential in energy production (e.g., cellular respiration), corrosion, and many industrial processes.
Beyond Simple Ionization: More Complex Scenarios
The discussion thus far has focused on single electron transfers. However, atoms can gain or lose multiple electrons, resulting in ions with higher charges. For example, magnesium (Mg) readily loses two electrons to form Mg²⁺, while aluminum (Al) loses three electrons to form Al³⁺. Similarly, some atoms can form polyatomic ions, where multiple atoms are bonded together and carry a net charge, such as sulfate (SO₄²⁻) and phosphate (PO₄³⁻).
Furthermore, the process of ionization can be influenced by external factors like:
- Temperature: Higher temperatures can provide the necessary energy to overcome the ionization energy and facilitate electron loss.
- Radiation: Exposure to high-energy radiation, such as X-rays or gamma rays, can ionize atoms by stripping away electrons.
- Electric fields: Strong electric fields can accelerate charged particles, leading to collisions and ionization.
Conclusion: A Fundamental Process with Far-Reaching Effects
The gain or loss of electrons, leading to the formation of ions, is a fundamental process with far-reaching consequences. It underpins a vast array of chemical and physical phenomena, from the formation of ionic compounds and the conduction of electricity to the intricate workings of biological systems and the energy transformations driving countless processes in the universe. A deep understanding of ionization is crucial for advancements in many scientific and technological fields. From developing new materials with specific properties to improving our understanding of biological processes and creating more efficient energy sources, the implications of this simple yet profound process remain a constant area of ongoing research and innovation. The seemingly small act of an electron transferring has enormous consequences on a macroscopic scale, highlighting the interconnectedness and complexity of the natural world.
Latest Posts
Latest Posts
-
How To Set Up A Vacuum Filtration
Apr 02, 2025
-
Chemistry Structure And Properties Nivaldo J Tro
Apr 02, 2025
-
Identify Which Criteria Are Used To Characterize Bacterial Colonies
Apr 02, 2025
-
Which Is The Longest Phase Of The Cell Cycle
Apr 02, 2025
-
Is Flammability A Chemical Or Physical Property
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Happens When An Atom Gains Or Loses An Electron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
