What Is A Calorie In Chemistry
Muz Play
Mar 26, 2025 · 5 min read
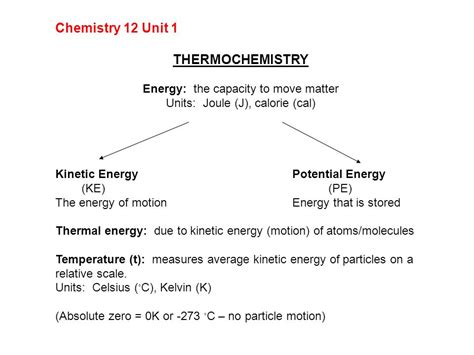
Table of Contents
What is a Calorie in Chemistry? A Deep Dive into Energy Measurement
Understanding calories is crucial, not just for dieters, but for anyone interested in chemistry, biology, and even physics. While often associated with weight loss and nutrition, a calorie's true nature lies within the realm of chemistry – specifically, as a unit of energy. This comprehensive guide explores the chemical definition of a calorie, its relationship to other energy units, its role in metabolic processes, and the misconceptions surrounding its use in everyday life.
Defining the Calorie: More Than Just Diet Talk
The term "calorie" itself can be confusing due to its multiple meanings. In everyday conversation, we typically refer to a "Calorie" (with a capital "C"), which is actually a kilocalorie (kcal). A kilocalorie represents 1000 calories. To truly understand a calorie, we must delve into its fundamental chemical definition.
A calorie (cal) is defined as the amount of heat energy required to raise the temperature of one gram of water by one degree Celsius (or one Kelvin). This definition is rooted in the specific heat capacity of water, a crucial property in chemistry and thermodynamics. This means that the calorie is a unit of energy transfer, specifically heat energy.
The Chemical Basis of Energy Measurement
The energy stored within chemical bonds is the foundation of the calorie. When bonds are broken, energy is released, often as heat. This is the basis of combustion reactions, where substances react with oxygen to produce heat and other products. The calorie allows us to quantify this released energy.
For example, the combustion of glucose (a simple sugar) releases a significant amount of energy:
C₆H₁₂O₆(s) + 6O₂(g) → 6CO₂(g) + 6H₂O(l) + energy
This energy is measured in calories, reflecting the heat produced during the reaction. This energy release fuels our bodies' metabolic processes. Understanding the chemical reactions involved in metabolism helps clarify how food provides energy measured in calories.
Calories and Metabolic Processes: The Body's Energy Engine
Our bodies are complex chemical reactors. The food we consume undergoes various chemical reactions (metabolism) to break down complex molecules into simpler ones, releasing energy in the process. This energy, measured in calories, powers bodily functions, from breathing and heartbeats to muscle movement and brain activity.
Macronutrients and Caloric Content:
The caloric content of food is primarily derived from three macronutrients:
- Carbohydrates: These complex molecules break down into glucose, providing readily available energy. One gram of carbohydrate provides approximately 4 calories.
- Proteins: These are essential for building and repairing tissues, but they also contribute to energy production. One gram of protein also provides approximately 4 calories.
- Fats: These are highly energy-dense molecules; one gram of fat provides approximately 9 calories. Fats serve vital functions in the body beyond energy storage.
The calorie count of a food item is a summation of the calories contributed by each macronutrient present, along with minor contributions from other substances.
Metabolism and Energy Expenditure:
The body doesn't just passively absorb calories. It uses energy constantly for various functions. Basal metabolic rate (BMR) represents the minimum energy required to keep the body functioning at rest. Physical activity, digestion, and other processes further increase energy expenditure.
A calorie deficit (consuming fewer calories than expended) leads to weight loss, as the body uses stored energy reserves (fat) to meet its energy demands. Conversely, a calorie surplus results in weight gain, as excess energy is stored as fat.
Calories and Other Energy Units: Conversions and Context
While the calorie is a common unit, particularly in the context of nutrition and metabolism, other energy units are frequently used in scientific contexts. Understanding the relationships between these units is essential:
- Joule (J): The SI unit of energy, the joule is the preferred unit in many scientific fields. One calorie is equivalent to approximately 4.184 joules.
- Kilocalorie (kcal): As mentioned earlier, this is the unit often referred to as a "Calorie" in dietary contexts. One kcal equals 1000 calories.
- Kilowatt-hour (kWh): This unit is commonly used for measuring electrical energy. It's a larger unit than the calorie, reflecting the energy consumption of larger systems.
The conversion between these units is straightforward, but using the appropriate unit for the context is crucial for clarity and accuracy.
Misconceptions about Calories: Separating Fact from Fiction
Several misconceptions surround the concept of calories:
- All calories are equal: This is false. While the caloric content of food indicates the energy it provides, the type of food and its impact on metabolism and satiety vary considerably. For instance, consuming 100 calories from refined sugar versus 100 calories from fruits will have different impacts on health.
- Calories are the only factor in weight management: Weight management is complex and involves factors beyond calorie intake and expenditure. Hormones, genetics, and overall lifestyle play a significant role.
- Counting calories is the only way to lose weight: While calorie counting can be a useful tool, it's not a magic bullet. Focusing on nutrient-rich foods and a balanced lifestyle is more sustainable.
Conclusion: The Chemical Heart of Calorie Counting
The seemingly simple concept of a calorie reveals a rich chemical and biological context. Understanding the underlying chemical principles involved in energy transfer, metabolic processes, and the relationship between calories and other energy units is fundamental to appreciating its significance in various scientific fields and daily life. While the calorie is a useful tool for understanding energy intake and expenditure, a holistic approach to health and nutrition goes beyond simple calorie counting. Remember to consult with healthcare professionals for personalized advice on diet and weight management. A balanced understanding of chemistry, biology, and mindful lifestyle choices contributes to overall wellbeing.
Latest Posts
Latest Posts
-
Find The Equation Of A Line Shown
Mar 29, 2025
-
Why Does Active Transport Require Energy
Mar 29, 2025
-
Cellulose And Starch Are Examples Of
Mar 29, 2025
-
What Is Procedural History In A Case Brief
Mar 29, 2025
-
Is Sweating Negative Or Positive Feedback
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is A Calorie In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
