What Is A Horizontal Row In The Periodic Table Called
Muz Play
Mar 20, 2025 · 6 min read
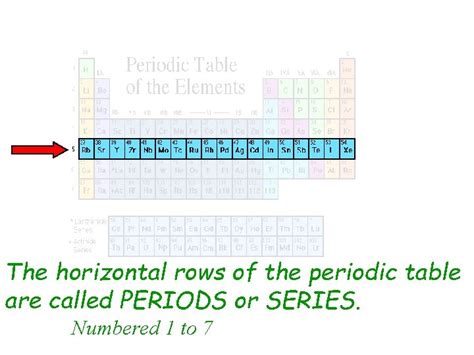
Table of Contents
What is a Horizontal Row in the Periodic Table Called? A Deep Dive into Periods and Their Significance
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding its organization is crucial to grasping chemical behavior and predicting reactions. One fundamental aspect of this organization is the arrangement of elements into horizontal rows. But what is a horizontal row in the periodic table actually called? The answer, simply put, is a period. This seemingly simple term encapsulates a wealth of information about the elements it contains and their interconnectedness. This article delves deep into the concept of periods, exploring their structure, properties, and significance in understanding the periodic table as a whole.
Understanding Periods: A Horizontal Journey Through Atomic Structure
A period in the periodic table represents a horizontal row of elements. Each period corresponds to a principal energy level (or shell) being filled with electrons. As we move across a period from left to right, the number of protons and electrons in the atoms increases progressively. This increase in atomic number leads to systematic changes in the elements' properties, creating recurring patterns which form the basis of the periodic law. The periodic law states that the properties of elements are periodic functions of their atomic numbers.
The Significance of Electron Shells and Periods
The number of periods corresponds directly to the number of electron shells that can be filled. The first period, for instance, contains only two elements: hydrogen (H) and helium (He). This is because the first energy level can only accommodate a maximum of two electrons. Similarly, the second period contains eight elements (lithium to neon) because the second energy level can hold a maximum of eight electrons. This pattern continues, albeit with increasing complexity, as we progress to higher periods.
Period Trends: A Systematic Shift in Properties
As we traverse a period, several key properties of elements exhibit predictable trends. These trends are a direct consequence of the increasing nuclear charge and the addition of electrons to the same principal energy level.
-
Atomic Radius: Generally, atomic radius decreases across a period. As we add protons to the nucleus, the increased positive charge pulls the electrons closer, resulting in a smaller atomic size.
-
Ionization Energy: Ionization energy, the energy required to remove an electron from an atom, increases across a period. The stronger attraction between the nucleus and electrons makes it more difficult to remove an electron.
-
Electronegativity: Electronegativity, the tendency of an atom to attract electrons in a chemical bond, increases across a period. The increased nuclear charge makes the atom more effective at attracting shared electrons.
-
Metallic Character: Metallic character, characterized by properties like conductivity and malleability, generally decreases across a period. Elements at the beginning of a period (alkali and alkaline earth metals) exhibit strong metallic properties, while those at the end (halogens and noble gases) have significantly weaker metallic character.
A Detailed Look at Each Period
Let's examine each period in detail to understand the unique characteristics of the elements they contain:
Period 1: The Shortest Journey
Period 1, the shortest period, contains only hydrogen (H) and helium (He). These elements have only one electron shell, and exhibit starkly contrasting properties. Hydrogen is a reactive, non-metallic gas, while helium is an inert noble gas.
Period 2 and 3: The Rise of the s and p Blocks
Periods 2 and 3 are both short periods containing eight elements each. These periods mark the introduction of the s and p blocks of the periodic table. They display a wider range of properties compared to period 1, encompassing both metals and non-metals. The elements in these periods begin to showcase the clear trends in atomic radius, ionization energy, and electronegativity discussed earlier.
Period 4 and 5: Introducing the d Block – Transition Metals
Periods 4 and 5 are longer periods due to the introduction of the d block elements, known as transition metals. These metals are characterized by their variable oxidation states and the formation of colorful compounds. The properties of transition metals exhibit less pronounced trends compared to the s and p block elements, with gradual changes across the period.
Period 6 and 7: The f Block and the Lanthanides and Actinides
Periods 6 and 7 are the longest periods, encompassing the f block elements: the lanthanides and actinides. These elements fill the 4f and 5f orbitals, respectively, and are largely similar in their chemical properties due to the shielding effect of the electrons in the f orbitals. The actinides, in particular, are known for their radioactivity.
The Significance of Periods in Chemical Bonding and Reactivity
The position of an element within a period dictates its reactivity and how it forms chemical bonds. Elements on the far left of a period (alkali metals) readily lose electrons to form positive ions, while elements on the far right (halogens) readily gain electrons to form negative ions. The elements in between exhibit varying degrees of electron donating or accepting tendencies. This difference in electron behavior drives the diverse chemical reactions observed across the periodic table.
Periods and the Periodic Law: A Cyclical Nature of Properties
The concept of periods is intrinsically linked to the periodic law. The recurring patterns in properties observed as we move across a period and then down to the next are what define the periodic nature of elements. This cyclical nature is a manifestation of the regular filling of electron shells and the consequential changes in the elements' electronic configurations.
Practical Applications and Further Exploration
Understanding periods is crucial in various fields, including:
-
Predicting chemical reactions: Knowledge of period trends enables scientists to anticipate the behavior of elements and predict the outcome of reactions.
-
Material science: Designing new materials with specific properties often involves selecting elements from particular periods based on their desired characteristics.
-
Nuclear chemistry: The periodic table, especially the actinide series in period 7, is essential for understanding radioactive decay and nuclear reactions.
-
Environmental chemistry: The properties of elements in different periods influence their environmental behavior and potential impact on ecosystems.
The exploration of periods within the periodic table extends beyond the basic trends and properties. Researchers continuously investigate the intricacies of electron configuration, bonding behavior, and reactivity of elements within specific periods. Further research explores the subtle nuances and exceptions to the general trends. This ongoing research deepens our understanding of the fundamental principles governing chemical interactions and facilitates advancements across various scientific disciplines.
In conclusion, understanding what a horizontal row in the periodic table is called – a period – is fundamental to understanding the organization and behavior of elements. The concept of periods encapsulates the systematic variations in elemental properties, driven by the progressive filling of electron shells and the resulting changes in electron configuration. This knowledge forms the bedrock for many advancements in chemistry and related fields, impacting our ability to predict reactions, design new materials, and understand the intricate workings of the natural world.
Latest Posts
Latest Posts
-
Media And Culture An Introduction To Mass Communication
Mar 20, 2025
-
How Do Animals Get Their Energy
Mar 20, 2025
-
How To Find Molecular Formula From Mass Spectrum
Mar 20, 2025
-
Do Bases And Acids React With Metals
Mar 20, 2025
-
Concentration Of A Sodium Chloride Solution
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Is A Horizontal Row In The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
