What Is A Lone Pair Of Electrons
Muz Play
Mar 22, 2025 · 6 min read
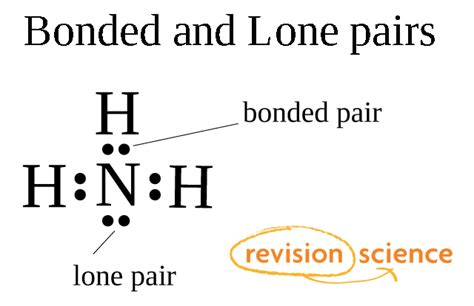
Table of Contents
What is a Lone Pair of Electrons? A Deep Dive into Chemical Bonding
Understanding the intricacies of chemical bonding is fundamental to grasping the behavior of matter. Central to this understanding is the concept of the lone pair of electrons. This seemingly simple idea plays a crucial role in determining molecular geometry, polarity, and reactivity. This comprehensive article will explore lone pairs in detail, covering their definition, identification, implications for molecular structure, and their impact on various chemical properties.
Defining Lone Pairs: Electrons Uninvolved in Bonding
A lone pair, also known as a non-bonding pair or unshared pair, refers to a pair of valence electrons that are not involved in covalent bonding. Unlike bonding pairs, which are shared between two atoms to form a chemical bond, lone pairs are associated with a single atom. They occupy specific atomic orbitals and significantly influence the overall electronic structure and geometry of a molecule.
Think of it this way: atoms want to achieve a stable electron configuration, often following the octet rule (eight valence electrons). They can achieve this by sharing electrons with other atoms (forming covalent bonds) or by possessing lone pairs. Lone pairs contribute to this stability, even though they are not directly involved in linking atoms together.
Key characteristics of lone pairs:
- Non-bonding: They do not participate in the formation of a chemical bond with another atom.
- Valence electrons: They are located in the outermost electron shell of an atom.
- Occupy orbitals: They reside in specific atomic orbitals, influencing the electron density around the atom.
- Influence molecular shape: Their presence significantly impacts the molecule's three-dimensional structure.
- Affect reactivity: They can participate in chemical reactions as Lewis bases, donating electron density.
Identifying Lone Pairs: Lewis Structures and VSEPR Theory
Identifying lone pairs requires a solid understanding of Lewis structures and the Valence Shell Electron Pair Repulsion (VSEPR) theory.
Lewis Structures: Visualizing Electron Distribution
Lewis structures, also known as Lewis dot diagrams, are simple representations of molecules that show the valence electrons of each atom and how they are arranged in bonds and lone pairs. To draw a Lewis structure:
- Count valence electrons: Determine the total number of valence electrons for all atoms in the molecule.
- Identify central atom: Usually, the least electronegative atom is placed in the center.
- Form single bonds: Connect each surrounding atom to the central atom with a single bond (two electrons).
- Satisfy the octet rule: Distribute the remaining electrons as lone pairs to fulfill the octet rule for each atom (except for hydrogen, which only needs two electrons).
- Multiple bonds: If necessary, form double or triple bonds to satisfy the octet rule.
Example: Consider water (H₂O). Oxygen has six valence electrons, and each hydrogen has one. The total is eight. Oxygen forms single bonds with each hydrogen, using four electrons. The remaining four electrons are distributed as two lone pairs on the oxygen atom.
VSEPR Theory: Predicting Molecular Geometry
VSEPR theory predicts the three-dimensional arrangement of atoms in a molecule based on the repulsion between electron pairs (both bonding and lone pairs) around the central atom. Electron pairs, whether bonding or lone pairs, repel each other and try to position themselves as far apart as possible to minimize repulsion. This determines the molecular geometry. Lone pairs exert a stronger repulsive force than bonding pairs, influencing the bond angles and overall shape.
Different electron pair arrangements and their impact on geometry:
- AX₂: Two bonding pairs, linear geometry (e.g., BeCl₂).
- AX₃: Three bonding pairs, trigonal planar geometry (e.g., BF₃).
- AX₄: Four bonding pairs, tetrahedral geometry (e.g., CH₄).
- AX₂E: Two bonding pairs and one lone pair, bent geometry (e.g., H₂O).
- AX₃E: Three bonding pairs and one lone pair, trigonal pyramidal geometry (e.g., NH₃).
- AX₂E₂: Two bonding pairs and two lone pairs, bent geometry (e.g., H₂S).
The presence of lone pairs significantly affects the geometry; they push the bonding pairs closer together, resulting in bond angles smaller than expected in the ideal geometry.
Implications of Lone Pairs: Molecular Properties and Reactivity
The presence and arrangement of lone pairs profoundly influence several molecular properties:
Molecular Polarity
Lone pairs contribute to molecular polarity. A polar molecule has a net dipole moment, meaning it has a positive and negative end due to an uneven distribution of electron density. Lone pairs, being regions of high electron density, create a partial negative charge on the atom possessing them. This uneven distribution can lead to a polar molecule, even if the individual bonds are nonpolar. Water (H₂O) is a classic example; the lone pairs on oxygen contribute to its high polarity.
Molecular Shape and Bond Angles
As previously discussed, lone pairs significantly influence the molecular shape and bond angles. They repel bonding pairs, causing deviations from ideal geometries and reducing bond angles. For example, in methane (CH₄), the bond angles are 109.5° (tetrahedral), but in water (H₂O), the bond angle is 104.5° due to the repulsion from the two lone pairs on oxygen.
Reactivity: Lewis Bases
Lone pairs act as Lewis bases, meaning they can donate electrons to electron-deficient species (Lewis acids). This ability to donate electron density is crucial for many chemical reactions. For instance, ammonia (NH₃) with its lone pair on nitrogen acts as a Lewis base in many reactions.
Lone Pairs in Different Chemical Contexts
The role and influence of lone pairs vary depending on the specific chemical context:
Organic Chemistry: Functional Groups and Reactivity
Lone pairs are crucial in organic chemistry, defining the reactivity and properties of various functional groups such as alcohols (-OH), amines (-NH₂), and ethers (-O-). The lone pairs on oxygen and nitrogen atoms in these functional groups allow them to participate in hydrogen bonding, influencing their solubility and boiling points. They also participate in many important reactions like nucleophilic attacks.
Inorganic Chemistry: Coordination Complexes
In inorganic chemistry, lone pairs are critical in the formation of coordination complexes. Ligands, molecules or ions that bind to a central metal atom, often possess lone pairs that they donate to the metal, forming coordinate covalent bonds. The number and nature of the lone pairs in the ligand influence the stability and properties of the coordination complex.
Physical Chemistry: Spectroscopic Techniques
The presence of lone pairs can be detected and studied using various spectroscopic techniques like infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy. The characteristic vibrational frequencies in IR and chemical shifts in NMR can provide valuable information about the presence and environment of lone pairs.
Conclusion: The Unsung Heroes of Chemical Bonding
Lone pairs of electrons, although not directly involved in forming chemical bonds, are fundamental to understanding molecular structure, properties, and reactivity. Their influence on molecular geometry, polarity, and reactivity is substantial, impacting various aspects of chemistry, from simple molecules to complex biological systems. A thorough understanding of lone pairs is crucial for anyone aiming to delve deeply into the fascinating world of chemical bonding and molecular interactions. By mastering the concepts of Lewis structures, VSEPR theory, and the impact of lone pairs, one can predict and interpret the behavior of a wide range of chemical substances. This knowledge forms the bedrock for advancements in various chemical fields, from materials science to drug discovery.
Latest Posts
Latest Posts
-
Moment Of Inertia Of A Thin Rod
Mar 22, 2025
-
What Is The Role Of Light In Photosynthesis
Mar 22, 2025
-
During Which Phase Of Mitosis Do Sister Chromatids Separate
Mar 22, 2025
-
What Is A Reasoning In Science
Mar 22, 2025
-
Is Speed And Sound Of Density Proportional Or Nonproportional
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about What Is A Lone Pair Of Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
