What Is A Negatively Charged Ion Called
Muz Play
Mar 25, 2025 · 6 min read
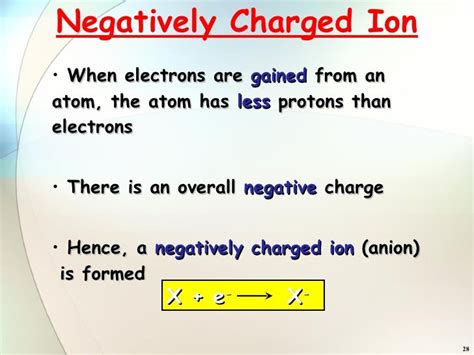
Table of Contents
What is a Negatively Charged Ion Called? A Deep Dive into Anions and Their Significance
Negatively charged ions, also known as anions, are ubiquitous in our environment and play crucial roles in various natural processes and technological applications. Understanding their properties, formation, and effects is essential across diverse scientific fields. This comprehensive guide delves into the world of anions, exploring their definition, characteristics, examples, and significance in different contexts.
Defining Anions: The Fundamentals of Negative Charge
At the heart of understanding negatively charged ions lies the concept of ionization. Atoms, in their neutral state, contain an equal number of protons (positively charged particles) and electrons (negatively charged particles). Ionization occurs when an atom either gains or loses electrons, disrupting this balance. When an atom gains one or more electrons, it acquires a net negative charge, transforming it into an anion. The process of anion formation is often associated with the atom's electronegativity – its tendency to attract electrons. Highly electronegative atoms readily accept electrons and form stable anions.
The name "anion" itself is derived from the Greek word "ana," meaning "up," and "ion," referring to the moving particle. This historical nomenclature reflects the early observations of ionic movement in electrolytic solutions, where negatively charged ions migrated towards the positive electrode (anode).
Key Characteristics of Anions
Anions exhibit several key characteristics that distinguish them from their positively charged counterparts (cations):
- Negative Charge: The defining characteristic is their net negative charge, resulting from an excess of electrons compared to protons. This charge magnitude can vary depending on the number of electrons gained.
- Size: Generally, anions are larger than their corresponding neutral atoms due to the addition of electrons. This increased electron-electron repulsion leads to expansion of the electron cloud.
- Reactivity: The reactivity of anions depends on several factors, including their size, charge, and the elements involved. Some anions are highly reactive, readily participating in chemical reactions, while others are relatively inert.
- Solubility: Anion solubility in different solvents varies significantly. Factors influencing solubility include the anion's charge density, size, and the polarity of the solvent.
- Conductivity: Anions contribute to the electrical conductivity of solutions and materials. Their mobility in solution allows them to carry electrical current.
Common Examples of Anions: A Diverse Spectrum
Anions encompass a wide array of chemical species, ranging from simple monatomic ions to complex polyatomic ions. Here are some notable examples categorized for clarity:
Monatomic Anions: Simple and Significant
These anions are formed from single atoms gaining electrons. Examples include:
- Chloride ion (Cl⁻): Found extensively in table salt (NaCl) and biological systems, playing crucial roles in electrolyte balance and nerve impulse transmission.
- Sulfide ion (S²⁻): A key component in many metal sulfide minerals and involved in various industrial processes.
- Oxide ion (O²⁻): A fundamental anion in numerous inorganic compounds and oxides, often forming the backbone of many mineral structures.
- Nitride ion (N³⁻): Present in nitrides, a class of compounds with diverse applications in materials science.
- Fluoride ion (F⁻): Added to drinking water to prevent tooth decay, showcasing its importance in public health.
Polyatomic Anions: Complexity and Functionality
Polyatomic anions consist of multiple atoms covalently bonded together carrying a net negative charge. This group demonstrates greater chemical complexity and a wider range of functionalities:
- Hydroxide ion (OH⁻): A fundamental anion in many chemical reactions, particularly acid-base chemistry, and present in alkaline solutions.
- Nitrate ion (NO₃⁻): A vital nutrient for plant growth, found extensively in fertilizers and present in many naturally occurring minerals.
- Sulfate ion (SO₄²⁻): Commonly found in minerals like gypsum and involved in various industrial processes and environmental chemistry.
- Phosphate ion (PO₄³⁻): Essential for life, playing critical roles in energy transfer and DNA structure. Found in fertilizers and many biological systems.
- Carbonate ion (CO₃²⁻): A crucial component of limestone and other carbonate rocks, involved in carbon cycling and geological processes.
- Acetate ion (CH₃COO⁻): A common anion in organic chemistry and utilized in various industrial and biological processes.
Formation of Anions: Mechanisms and Processes
The formation of anions involves the transfer or sharing of electrons. Several key mechanisms contribute to this process:
- Electron Transfer: This is the most straightforward mechanism where an atom directly gains one or more electrons from another atom, resulting in the formation of a negatively charged anion. This often happens in reactions between metals and non-metals.
- Covalent Bond Formation: In polyatomic anions, covalent bonds form between atoms, but the overall molecule carries a net negative charge due to an imbalance in the number of protons and electrons. The electronegativity differences between the atoms dictate the electron distribution and the resultant charge.
- Acid-Base Reactions: In many acid-base reactions, the base accepts a proton (H⁺), effectively creating a conjugate base which is an anion. For instance, when a hydroxide ion (OH⁻) reacts with an acid, the acid donates a proton, forming water (H₂O) and the conjugate base.
- Dissociation of Ionic Compounds: When soluble ionic compounds dissolve in water, they dissociate into their constituent ions, including anions. For example, table salt (NaCl) dissociates into Na⁺ and Cl⁻ ions in water.
Significance of Anions Across Diverse Fields
The impact of anions extends far beyond the realm of chemistry. Their importance spans various scientific disciplines and technological applications:
Biological Systems: The Foundation of Life
Anions are integral to countless biological processes. Examples include:
- Electrolyte Balance: Anions like chloride and bicarbonate contribute significantly to maintaining the proper electrolyte balance in bodily fluids.
- Enzyme Activity: Many enzymes require the presence of specific anions for their optimal functioning.
- Nerve Impulse Transmission: Chloride ions are crucial in regulating nerve impulse transmission.
- Bone Structure: Phosphate and other anions are essential components of bone structure.
- DNA and RNA: Phosphate anions are fundamental building blocks of DNA and RNA, the carriers of genetic information.
Environmental Science: Shaping Our World
Anions play crucial roles in shaping our environment:
- Water Quality: The presence and concentration of various anions affect water quality, impacting its suitability for drinking and other uses.
- Soil Chemistry: Anions like nitrates and phosphates influence soil fertility and plant growth.
- Acid Rain: Sulfate and nitrate anions are significant contributors to acid rain, impacting ecosystems and infrastructure.
- Atmospheric Chemistry: Anions participate in various atmospheric reactions, influencing air quality and climate.
Industrial Applications: Driving Technological Advancements
Anions are employed in a wide array of industrial applications:
- Material Science: Anions are used in the synthesis of numerous materials with specific properties, from ceramics to polymers.
- Catalysis: Certain anions act as catalysts in various industrial processes, speeding up reactions and improving efficiency.
- Electrochemistry: Anions participate in electrochemical processes, powering batteries and other electrochemical devices.
- Food Industry: Some anions are used as food additives, preserving food and enhancing its quality.
- Medicine: Certain anions are used in pharmaceuticals and medical treatments, addressing various health conditions.
Conclusion: The Enduring Importance of Anions
Negatively charged ions, or anions, are fundamental chemical species with profound implications across various fields. Their properties, formation, and interactions shape our understanding of biological processes, environmental dynamics, and technological advancements. From the intricate mechanisms within our bodies to the vast scale of geological formations and industrial processes, anions represent an essential aspect of the natural world and human innovation. Further research into anion behavior and interactions holds the key to unlocking further possibilities in medicine, materials science, and environmental management. Understanding anions is not merely an academic pursuit; it's a critical element in unraveling the complexities of our world and shaping a better future.
Latest Posts
Latest Posts
-
Vice Of Excess And Deficiency Virtue Chart Scrupulosity Laxity
Mar 25, 2025
-
How Many Are In A Set
Mar 25, 2025
-
Biology Word That Starts With J
Mar 25, 2025
-
What Is The Instrument To Measure Humidity
Mar 25, 2025
-
El Preterito De Los Verbos Regulares
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is A Negatively Charged Ion Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
