What Is A Polymer Of Proteins
Muz Play
Mar 17, 2025 · 6 min read
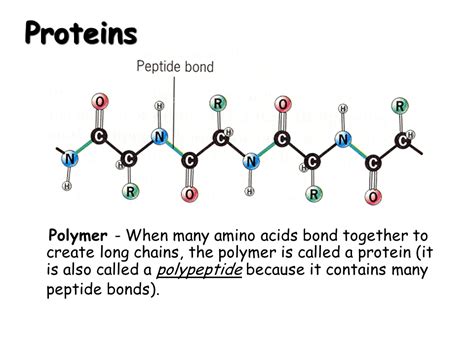
Table of Contents
What is a Polymer of Proteins? Understanding the Building Blocks of Life
Proteins are the workhorses of the cell, carrying out a vast array of functions essential for life. From catalyzing biochemical reactions to providing structural support, their versatility stems from their complex structures, which are ultimately determined by their building blocks: amino acids. But what exactly is a protein, in terms of its polymeric nature? This article delves deep into the polymeric structure of proteins, exploring the intricacies of amino acid linkages, the different levels of protein structure, and the implications of their polymeric nature for biological function.
Amino Acids: The Monomers of Protein Polymers
Proteins are polymers, meaning they are large molecules composed of repeating smaller units called monomers. In the case of proteins, these monomers are amino acids. There are 20 standard amino acids, each with a unique side chain (R-group) that dictates its chemical properties. These properties are crucial in determining the overall structure and function of the resulting protein.
The Structure of an Amino Acid
Each amino acid shares a common basic structure:
- Amino group (-NH2): A nitrogen-containing group that acts as a base.
- Carboxyl group (-COOH): A carbon-containing group that acts as an acid.
- α-carbon: A central carbon atom to which the amino group, carboxyl group, and R-group are attached.
- R-group (side chain): This varies among the 20 standard amino acids, imparting unique chemical characteristics such as hydrophobicity, hydrophilicity, charge, and size.
This seemingly simple structure is the key to the amazing diversity and functionality of proteins.
Peptide Bonds: Linking Amino Acids Together
Amino acids link together to form proteins through a peptide bond. This bond is a covalent bond formed between the carboxyl group of one amino acid and the amino group of another. The reaction involves the removal of a water molecule (dehydration synthesis), creating a peptide linkage (-CO-NH-) between the two amino acids. A chain of amino acids linked by peptide bonds is called a polypeptide. Proteins are essentially one or more polypeptides folded into a specific three-dimensional structure.
The Levels of Protein Structure
The complexity of protein structure can be categorized into four levels: primary, secondary, tertiary, and quaternary. Each level builds upon the previous one, ultimately determining the protein's function.
1. Primary Structure: The Amino Acid Sequence
The primary structure of a protein is simply the linear sequence of amino acids in the polypeptide chain. This sequence is determined by the genetic code encoded in DNA. Even a single amino acid change can drastically alter the protein's function, as seen in sickle cell anemia, where a single amino acid substitution in hemoglobin leads to a debilitating disease. The primary structure dictates all subsequent levels of protein organization. It is the foundation upon which the entire protein structure is built. Understanding this sequence is fundamental to understanding the protein's behavior and function. Changes at this level can have profound consequences, highlighting the crucial importance of maintaining the precise amino acid order.
2. Secondary Structure: Local Folding Patterns
The secondary structure refers to local folding patterns within the polypeptide chain, stabilized by hydrogen bonds between the backbone atoms (carbonyl oxygen and amide hydrogen). Common secondary structures include:
- α-helices: A right-handed coiled structure stabilized by hydrogen bonds between every fourth amino acid.
- β-sheets: Extended polypeptide chains arranged side-by-side, forming a pleated sheet structure stabilized by hydrogen bonds between adjacent strands.
- Loops and turns: Irregular regions connecting α-helices and β-sheets.
The specific secondary structures adopted by a protein depend on the amino acid sequence and the local environment. These local structures are crucial for creating the overall three-dimensional shape of the protein. Understanding the interactions that stabilize these formations is vital for comprehending protein stability and function.
3. Tertiary Structure: The 3D Conformation
The tertiary structure refers to the overall three-dimensional arrangement of a polypeptide chain. This structure is determined by interactions between the side chains (R-groups) of the amino acids. These interactions can include:
- Hydrophobic interactions: Nonpolar side chains cluster together in the protein's interior, away from the aqueous environment.
- Hydrogen bonds: Bonds formed between polar side chains.
- Ionic bonds (salt bridges): Electrostatic attractions between oppositely charged side chains.
- Disulfide bonds: Covalent bonds formed between cysteine residues.
The tertiary structure is crucial for the protein's function. The specific arrangement of amino acid side chains creates a unique active site for enzymes, a binding site for receptors, or a structural feature for support proteins. The intricate folding process is often assisted by chaperone proteins, ensuring the protein folds correctly and avoids misfolding, which can lead to aggregation and dysfunction. This folding is influenced by various factors, including temperature, pH, and the presence of other molecules.
4. Quaternary Structure: Multiple Polypeptide Chains
Some proteins consist of multiple polypeptide chains, each with its own tertiary structure, assembled into a functional complex. This arrangement is called the quaternary structure. The individual polypeptide chains, often called subunits, are held together by the same types of interactions that stabilize tertiary structure. Examples of proteins with quaternary structure include hemoglobin and many enzymes. The specific arrangement and interactions between subunits are crucial for the overall function of the protein complex, often enabling cooperative binding or allosteric regulation.
The Importance of Protein Polymerization
The polymeric nature of proteins is crucial for several reasons:
- Diversity: The ability to combine 20 different amino acids in various sequences allows for a vast number of possible protein structures and functions.
- Specificity: The precise sequence and three-dimensional arrangement of amino acids determine the protein's unique ability to interact with other molecules.
- Regulation: Protein structure and function can be regulated through various mechanisms, including post-translational modifications, protein-protein interactions, and allosteric regulation.
- Evolution: The modularity of protein structures allows for the evolution of new functions through gene duplication and mutation. Slight alterations in amino acid sequences can lead to significant changes in protein function, contributing to the adaptability of organisms.
The Impact of Polymerization on Protein Function
The polymeric structure directly influences how proteins perform their roles. For instance, enzymes rely on the precisely positioned amino acids in their active sites to catalyze specific reactions. Structural proteins, like collagen, utilize the repeating units and strong interactions within the polypeptide chains to provide support and strength to tissues. Transport proteins, such as hemoglobin, take advantage of the quaternary structure to efficiently bind and release oxygen.
Protein Misfolding and Diseases
The correct folding of proteins is essential for their function. Errors in folding can lead to the formation of misfolded proteins, which can aggregate and form amyloid fibrils. These aggregates are associated with various diseases, including Alzheimer's disease, Parkinson's disease, and Huntington's disease. Understanding the mechanisms of protein folding and misfolding is crucial for developing therapeutic strategies for these diseases.
Conclusion
Proteins are remarkable polymers whose structure directly relates to their function. The precise arrangement of amino acids, from the primary sequence to the quaternary structure, is a testament to the power of polymeric organization in biology. Understanding the intricacies of protein structure is vital for comprehending the complexity of life and developing new therapies for diseases related to protein misfolding. Further research continues to reveal the subtle nuances of protein folding, interactions, and regulation, enriching our understanding of this fundamental biological building block. The remarkable diversity and sophistication of protein polymers underscore their critical role in virtually every aspect of cellular processes and organismal life. From the simplest bacteria to the most complex mammals, proteins, in all their polymeric glory, serve as the molecular machinery driving life's intricate dance.
Latest Posts
Latest Posts
-
Which Body Cavity Protects The Spinal Column
Mar 17, 2025
-
Sampling Distribution Of The Sample Mean Calculator
Mar 17, 2025
-
Expected Value Of A Discrete Random Variable
Mar 17, 2025
-
Calculate Generation Time From Growth Curve
Mar 17, 2025
-
Factors That Affect Elasticity Of Supply
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is A Polymer Of Proteins . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
