What Is An Intermediate In A Reaction
Muz Play
Mar 30, 2025 · 5 min read
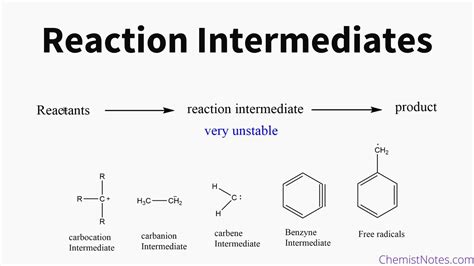
Table of Contents
What is an Intermediate in a Reaction? A Deep Dive into Reaction Mechanisms
Understanding reaction mechanisms is crucial in chemistry. A key component of these mechanisms is the intermediate. This article will provide a comprehensive exploration of intermediates, their characteristics, identification, and significance in various reaction types. We will delve into the nuances of their existence, differentiating them from other species involved in reactions, and highlight their importance in predicting reaction pathways and developing new chemical processes.
Defining Intermediates: Transient Species in Chemical Transformations
In a chemical reaction, an intermediate is a short-lived, high-energy species that is formed during the reaction but is not present in the overall stoichiometric equation. It's crucial to understand that intermediates are not reactants or products; they exist only transiently, forming and then reacting further before the reaction's completion. This transient nature makes their direct observation challenging, often requiring specialized techniques.
Think of a reaction mechanism as a road trip. The reactants are your starting point, the products your destination, and intermediates are the temporary stops along the way. You wouldn't include these stops in the overall description of your journey from origin to destination, but they are crucial parts of the actual trip.
Distinguishing Intermediates from Other Reaction Species
It's essential to differentiate intermediates from other species involved in chemical reactions:
1. Reactants vs. Intermediates
Reactants are the starting materials that are consumed during the reaction. They appear on the left side of the reaction equation. Intermediates, on the other hand, are generated during the reaction and subsequently consumed. They are neither reactants nor products in the overall balanced equation.
2. Products vs. Intermediates
Products are the final species formed at the end of the reaction and appear on the right side of the equation. Intermediates are transient species that react further to form the final products. They do not appear in the balanced equation.
3. Transition States vs. Intermediates
This is a crucial distinction. A transition state is a high-energy, unstable arrangement of atoms representing the maximum energy point along the reaction coordinate. It exists for an incredibly short time, even shorter than an intermediate. Unlike intermediates, transition states cannot be isolated or directly observed. They are theoretical constructs, used to explain the energy profile of a reaction. Intermediates, while short-lived, have a finite lifetime, albeit brief, and may, under certain conditions, be characterized spectroscopically.
Characteristics of Reaction Intermediates
Several key characteristics help identify potential intermediates:
- High energy: Intermediates possess higher energy than both reactants and products. This high energy contributes to their instability and short lifetime.
- Short lifetime: Their transient nature makes detection challenging. They react quickly to form other species.
- Not present in the stoichiometric equation: The overall reaction equation doesn't show intermediates because they are consumed during the reaction sequence.
- Often reactive: Their high energy means they readily participate in further reactions.
- May be characterized spectroscopically: Advanced techniques, like flash photolysis or rapid-scan spectroscopy, can sometimes capture evidence of intermediates.
Identifying Intermediates: Experimental and Theoretical Approaches
Pinpointing intermediates can be a significant challenge. Researchers employ various techniques:
1. Kinetic Studies:
Analyzing reaction rates provides insights into the steps involved. If a reaction displays multiple rate constants, it suggests a multi-step mechanism with intermediates.
2. Spectroscopic Techniques:
Techniques like infrared (IR), nuclear magnetic resonance (NMR), electron paramagnetic resonance (EPR), and UV-Vis spectroscopy can detect intermediates by identifying their unique spectral signatures. However, this often requires fast reaction monitoring capabilities.
3. Isotopic Labeling:
Using isotopes (e.g., deuterium, 13C) can help track atom movements during the reaction, revealing the formation and fate of intermediates.
4. Computational Chemistry:
Theoretical calculations, using programs like Gaussian or ORCA, can predict the structures and energies of possible intermediates, supporting experimental findings.
Examples of Intermediates in Different Reaction Types
Intermediates are ubiquitous in many reaction mechanisms. Let's examine some examples:
1. Nucleophilic Substitution (SN1 and SN2):
- SN1 (Unimolecular Nucleophilic Substitution): Involves the formation of a carbocation intermediate. The rate-determining step is the departure of the leaving group, generating this unstable intermediate.
- SN2 (Bimolecular Nucleophilic Substitution): Typically doesn't involve a discrete intermediate. The reaction proceeds through a single concerted step, where bond breaking and bond formation occur simultaneously.
2. Electrophilic Aromatic Substitution:
This mechanism involves the formation of a arenium ion intermediate, a positively charged species with a cyclohexadienyl structure. This intermediate is stabilized by resonance.
3. Addition Reactions (e.g., halogenation of alkenes):
Often involve the formation of a cyclic halonium ion intermediate, a three-membered ring containing a halogen atom. This intermediate is then attacked by a nucleophile.
4. Free Radical Reactions:
These reactions involve the formation of highly reactive free radical intermediates, species with unpaired electrons. Their high reactivity leads to chain propagation steps.
5. Enzyme-Catalyzed Reactions:
In biological systems, enzymes often facilitate reactions through the formation of enzyme-substrate complexes, which can be considered intermediates in the overall catalytic cycle.
The Significance of Intermediates in Reaction Mechanisms
Understanding intermediates is crucial for several reasons:
- Predicting reaction pathways: Knowing which intermediates form helps predict the overall reaction outcome.
- Developing new chemical processes: Identifying key intermediates can lead to the design of new synthetic routes with higher yields and selectivity.
- Improving reaction efficiency: Understanding the stability and reactivity of intermediates allows for optimization of reaction conditions.
- Catalyst design: The identification of intermediates provides valuable insights for designing new catalysts that can stabilize or destabilize specific intermediates, thereby influencing reaction rates and selectivity.
- Understanding biological processes: Intermediates play crucial roles in many biological reactions, understanding them is vital to understanding enzyme function and metabolic pathways.
Conclusion: Unraveling the Transient World of Reaction Intermediates
Intermediates, although fleeting, are essential components of reaction mechanisms. Their transient nature makes their investigation challenging, demanding sophisticated techniques and theoretical approaches. However, understanding their formation, properties, and reactivity is vital for deepening our comprehension of chemical transformations and manipulating them for various applications, from designing new drugs and materials to developing more efficient industrial processes. The continuing exploration of reaction intermediates remains a vibrant and crucial area of chemical research. Further advancements in experimental and computational methods promise to continue to shed light on the transient world of reaction intermediates, expanding our ability to control and exploit their remarkable properties.
Latest Posts
Latest Posts
-
Root Mean Square Velocity Of Gas
Apr 01, 2025
-
Where Is The Respiratory Center Located In The Brain
Apr 01, 2025
-
What Is The Difference Between Cellular Respiration And Fermentation
Apr 01, 2025
-
What Are Two Functional Groups Found In Amino Acids
Apr 01, 2025
-
Foundations Of Education 13th Edition Pdf Free Download
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is An Intermediate In A Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
