What Is The Difference Between Monatomic And Polyatomic Ions
Muz Play
Apr 07, 2025 · 6 min read
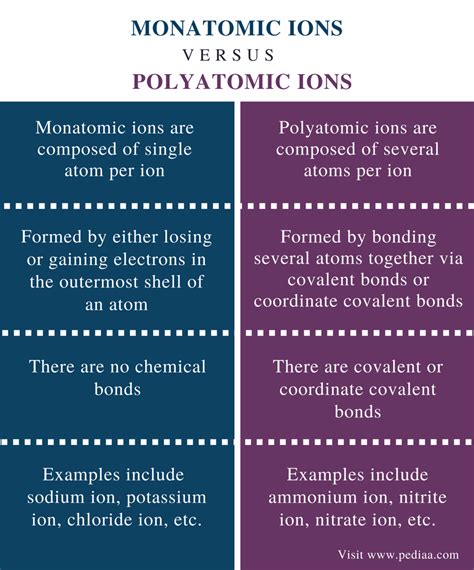
Table of Contents
What's the Difference Between Monatomic and Polyatomic Ions? A Deep Dive
Understanding the fundamental building blocks of chemistry is crucial for grasping more complex concepts. Ions, charged atoms or molecules, play a vital role in numerous chemical reactions and processes. This article delves deep into the distinction between two primary types of ions: monatomic ions and polyatomic ions. We'll explore their definitions, structures, properties, and examples, providing a comprehensive understanding of their differences and significance in the chemical world.
Defining Monatomic Ions: The Lone Atoms
Monatomic ions are single atoms that carry an electric charge. This charge arises from an imbalance between the number of protons (positively charged) and electrons (negatively charged) within the atom. When an atom loses electrons, it becomes positively charged, forming a cation. Conversely, when an atom gains electrons, it becomes negatively charged, forming an anion.
Formation of Monatomic Ions
The formation of monatomic ions is governed by the atom's electronic configuration and its tendency to achieve a stable electron configuration, often resembling that of a noble gas. This is explained by the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a full outer electron shell of eight electrons (or two for hydrogen and helium).
Let's consider the example of sodium (Na). Sodium has one electron in its outermost shell. To achieve a stable octet, it readily loses this single electron, forming a sodium cation (Na⁺). Conversely, chlorine (Cl) has seven electrons in its outermost shell. It readily gains one electron to complete its octet, forming a chloride anion (Cl⁻).
Key characteristics of monatomic ions:
- Single atom: They consist of only one atom.
- Charged: They carry a positive (cation) or negative (anion) charge.
- Simple structure: Their structure is straightforward, reflecting the electronic configuration of the parent atom.
- Predictable charge: The charge is often predictable based on the atom's position in the periodic table (e.g., alkali metals usually form +1 cations, halogens usually form -1 anions).
Examples of Monatomic Ions
Many common elements form monatomic ions. Here are a few examples:
- Cations: Na⁺ (sodium), K⁺ (potassium), Ca²⁺ (calcium), Mg²⁺ (magnesium), Al³⁺ (aluminum), Fe²⁺ (iron(II)), Fe³⁺ (iron(III))
- Anions: Cl⁻ (chloride), Br⁻ (bromide), I⁻ (iodide), O²⁻ (oxide), S²⁻ (sulfide), N³⁻ (nitride)
Delving into Polyatomic Ions: Groups of Atoms with a Charge
Unlike monatomic ions, polyatomic ions consist of two or more atoms covalently bonded together, carrying a net electric charge. This charge results from an imbalance of electrons within the group of atoms. These ions behave as single units in chemical reactions.
Formation of Polyatomic Ions
Polyatomic ions typically involve nonmetals bonded together, often with one atom acting as a central atom surrounded by other atoms. The bonding within the polyatomic ion is covalent, meaning atoms share electrons to achieve a stable electron configuration. The overall charge is determined by the difference between the total number of protons and electrons in the entire group.
For instance, the sulfate ion (SO₄²⁻) contains one sulfur atom and four oxygen atoms covalently bonded. The overall charge of -2 results from the distribution of electrons within the ion.
Key characteristics of polyatomic ions:
- Multiple atoms: They are composed of two or more atoms covalently bonded.
- Charged: They carry a net positive or negative charge.
- Complex structure: Their structure is more complex than that of monatomic ions.
- Variable charge: Their charge is not always easily predictable, requiring knowledge of the bonding and electron distribution within the ion.
- Covalent bonding within: The atoms are held together by covalent bonds.
Examples of Polyatomic Ions
Polyatomic ions are abundant in chemistry, playing significant roles in various compounds and reactions. Some common examples include:
- Anions: OH⁻ (hydroxide), NO₃⁻ (nitrate), SO₄²⁻ (sulfate), PO₄³⁻ (phosphate), CO₃²⁻ (carbonate), ClO⁻ (hypochlorite), ClO₂⁻ (chlorite), ClO₃⁻ (chlorate), ClO₄⁻ (perchlorate), CrO₄²⁻ (chromate), Cr₂O₇²⁻ (dichromate)
- Cations: NH₄⁺ (ammonium), H₃O⁺ (hydronium)
Comparing Monatomic and Polyatomic Ions: A Side-by-Side Look
| Feature | Monatomic Ions | Polyatomic Ions |
|---|---|---|
| Number of Atoms | One | Two or more |
| Bonding | No bonds within the ion | Covalent bonds between atoms |
| Charge | Positive (cation) or negative (anion) | Positive (cation) or negative (anion) |
| Structure | Simple; reflects electronic configuration | More complex; determined by bonding |
| Predictability of Charge | Often predictable from periodic table position | Less predictable; depends on bonding |
| Examples | Na⁺, Cl⁻, Ca²⁺, O²⁻ | OH⁻, SO₄²⁻, NO₃⁻, NH₄⁺, PO₄³⁻ |
The Importance of Ions in Chemical Reactions and Everyday Life
Both monatomic and polyatomic ions are essential components in a vast range of chemical reactions and everyday processes. They play crucial roles in:
- Electrolyte solutions: Many solutions conduct electricity because of the presence of ions. These solutions are essential in biological systems and numerous industrial applications.
- Chemical reactions: Ions participate in numerous chemical reactions, including acid-base reactions, redox reactions, and precipitation reactions.
- Biological systems: Ions are vital for biological processes such as nerve impulse transmission, muscle contraction, and enzyme activity. For example, sodium (Na⁺) and potassium (K⁺) ions are crucial for nerve impulse transmission.
- Industrial processes: Ions are utilized in various industrial processes, including electroplating, metal extraction, and water treatment.
Naming Ions: A System for Clarity
A systematic approach is used for naming ions to avoid confusion.
Naming Monatomic Ions
- Cations: For most metals, the name of the cation is the same as the element's name (e.g., sodium ion (Na⁺)). Transition metals can form multiple ions with different charges. In such cases, Roman numerals in parentheses indicate the charge (e.g., iron(II) ion (Fe²⁺), iron(III) ion (Fe³⁺)).
- Anions: The names of anions typically end in "-ide" (e.g., chloride (Cl⁻), sulfide (S²⁻), oxide (O²⁻)).
Naming Polyatomic Ions
The naming of polyatomic ions is more diverse and often requires memorization. Many common polyatomic ions have specific names, while others follow systematic naming conventions. For example, oxyanions (polyatomic ions containing oxygen) often have prefixes and suffixes to indicate the oxidation state of the central atom (e.g., chlorite (ClO₂⁻), chlorate (ClO₃⁻), perchlorate (ClO₄⁻)).
Conclusion: Understanding the Foundation
The distinction between monatomic and polyatomic ions is fundamental to understanding chemical reactions and various processes. While monatomic ions represent single charged atoms, polyatomic ions involve covalently bonded groups of atoms carrying a net charge. Their different structures, formation mechanisms, and properties contribute significantly to the complexity and diversity of chemical phenomena, playing essential roles in both natural and industrial settings. A firm grasp of these concepts forms a critical cornerstone in mastering chemistry.
Latest Posts
Latest Posts
-
Does Protists Reproduce Sexually Or Asexually
Apr 10, 2025
-
A Non Profit Organization Plans To Hold A Raffle
Apr 10, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Monatomic And Polyatomic Ions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.