What Is The Electron Configuration Of Boron
Muz Play
Apr 07, 2025 · 6 min read
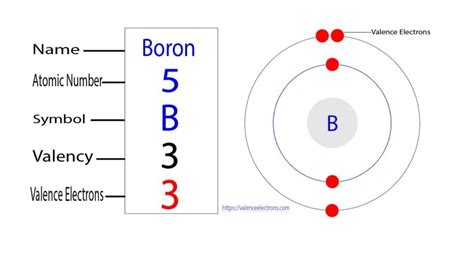
Table of Contents
What is the Electron Configuration of Boron? A Deep Dive into Atomic Structure
Boron, a metalloid element with the symbol B and atomic number 5, holds a significant place in chemistry and material science. Understanding its electron configuration is key to grasping its unique properties and reactivity. This comprehensive guide will explore the electron configuration of boron, delve into the principles behind its arrangement, and examine its implications for boron's chemical behavior.
Understanding Electron Configuration
Before we dive into the specifics of boron's electron configuration, let's establish a foundational understanding of what electron configuration actually means. An electron configuration describes the arrangement of electrons in the various energy levels and sublevels within an atom. This arrangement is governed by the principles of quantum mechanics, which dictate that electrons occupy specific orbitals characterized by distinct energy levels and shapes.
The electron configuration is typically represented using a shorthand notation, where the principal energy levels (n=1, 2, 3, etc.) are indicated by numbers, and the sublevels (s, p, d, f) are represented by letters. Each sublevel can accommodate a specific number of electrons:
- s sublevel: Holds a maximum of 2 electrons.
- p sublevel: Holds a maximum of 6 electrons.
- d sublevel: Holds a maximum of 10 electrons.
- f sublevel: Holds a maximum of 14 electrons.
The filling of these sublevels follows the Aufbau principle, which states that electrons fill the lowest energy levels first. This is followed by Hund's rule, which dictates that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. Finally, the Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers (n, l, ml, and ms).
Deriving Boron's Electron Configuration
Boron, with an atomic number of 5, possesses 5 electrons. To determine its electron configuration, we systematically fill the energy levels according to the Aufbau principle.
-
The first energy level (n=1): This level contains only the 1s sublevel, which can hold a maximum of 2 electrons. Therefore, boron's two lowest-energy electrons fill this sublevel completely.
-
The second energy level (n=2): This level comprises the 2s and 2p sublevels. The 2s sublevel, similar to the 1s, can accommodate 2 electrons. These two electrons fill the 2s sublevel completely.
-
The remaining electron: Boron has one electron remaining. This electron will occupy one of the three 2p orbitals. According to Hund's rule, it will occupy an orbital by itself before pairing up with another electron in the same orbital.
Therefore, the complete electron configuration of boron is 1s²2s²2p¹.
Visualizing Boron's Electron Configuration
To further solidify the understanding, let's visualize the electron configuration using orbital diagrams.
Each box represents an orbital, and each arrow represents an electron. The upward arrow signifies one spin state, and the downward arrow signifies the opposite spin state.
1s²: [ ↑↓ ]
2s²: [ ↑↓ ]
2p¹: [ ↑ ] [ ] [ ] (only one 2p orbital occupied)
This visualization clearly shows that boron has a completely filled 1s and 2s sublevel and a partially filled 2p sublevel with only one electron occupying one of the three available 2p orbitals.
Implications of Boron's Electron Configuration
Boron's electron configuration directly influences its chemical and physical properties. The presence of only one electron in the 2p sublevel means that boron readily forms covalent bonds by sharing this electron with other atoms. This tendency explains boron's relatively high reactivity, especially with electronegative elements like oxygen and halogens.
Covalent Bonding: Boron's incomplete 2p sublevel drives its propensity to form covalent bonds to achieve a more stable electron configuration. This is often reflected in its compounds, where boron shares electrons with other atoms to complete its valence shell. For instance, in boron trifluoride (BF₃), boron shares its three valence electrons with three fluorine atoms, forming three covalent bonds.
Formation of Borides: Boron also forms a variety of borides – compounds with other elements like transition metals. These borides often exhibit interesting and technologically relevant properties, such as high hardness and melting points, making them suitable for high-temperature applications. The electronic structure of boron significantly contributes to the bonding in these materials.
Electron Deficiency: The incomplete octet in boron's valence shell (only 3 valence electrons) leads to what's known as electron deficiency. This means that boron's compounds often exhibit Lewis acidity, meaning they can accept electron pairs from other species. This electron deficiency is responsible for the formation of electron-deficient bonds in boron compounds.
Unique Properties: Boron's electron configuration underpins many of its unique physical and chemical properties, including its relatively high melting point, its semi-conducting nature, and its ability to form diverse compounds with unique bonding structures.
Boron's Role in Different Fields
Understanding the electron configuration of boron is crucial in various fields. Its properties derived from its electronic structure make it vital in:
-
Materials Science: Boron's lightweight yet durable nature, combined with its high melting point, contributes to its use in high-strength materials like aerospace components and advanced composites.
-
Semiconductors: Boron's semi-conducting properties are leveraged in the electronics industry for the creation of p-type semiconductors, which are essential components of transistors and integrated circuits. Its electron configuration determines its capacity to accept electrons, contributing to its p-type behavior.
-
Nuclear Energy: Boron-10, an isotope of boron, has high neutron absorption cross-section. This property makes it valuable as a neutron absorber in nuclear reactors for controlling the nuclear fission reaction.
Advanced Concepts Related to Boron's Electron Configuration
While the basic electron configuration provides a fundamental understanding, more advanced concepts such as molecular orbital theory and hybridisation provide a more detailed description of boron's bonding behavior.
Molecular Orbital Theory: This theory considers the interaction of atomic orbitals to form molecular orbitals, which provide a more accurate representation of the electron distribution in molecules containing boron. It helps explain the bonding in more complex boron compounds and their unique properties.
Hybridisation: Hybridisation is a concept that describes the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies. Boron's sp² hybridization in compounds like BF₃ is crucial in understanding its trigonal planar geometry.
Conclusion: The Importance of Electron Configuration
The electron configuration of boron, 1s²2s²2p¹, is not just a simple notation; it's a key to unlocking its unique chemical and physical properties. From its tendency to form covalent bonds and act as a Lewis acid to its vital roles in materials science and nuclear technology, boron’s behavior is fundamentally shaped by its electronic structure. Understanding this fundamental aspect of boron's atomic structure provides a crucial foundation for appreciating its diverse applications and continuing research in this fascinating field. The detailed exploration of its electron configuration helps clarify its reactivity, bonding characteristics, and ultimate contributions to various technological advancements. Further study into advanced concepts like molecular orbital theory and hybridisation provides an even deeper understanding of this versatile element.
Latest Posts
Latest Posts
-
Ground State Electron Configuration For Calcium
Apr 09, 2025
-
How Is Hydrate Different From Other Chemical Compounds
Apr 09, 2025
-
Which Eukaryotic Cell Cycle Event Is Missing In Binary Fission
Apr 09, 2025
-
The Biological Approach To Psychological Disorders Focuses On
Apr 09, 2025
-
A Plane And A Line Can Intersect In A Point
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Boron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.