What Is The Horizontal Rows On The Periodic Table Called
Muz Play
Mar 24, 2025 · 7 min read
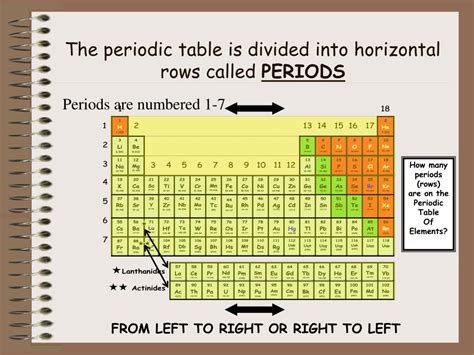
Table of Contents
What are the Horizontal Rows on the Periodic Table Called? A Deep Dive into Periods and Their Significance
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. While many are familiar with the vertical columns, called groups or families, understanding the horizontal rows, known as periods, is equally crucial for grasping the fundamental principles of chemistry. This article will delve deep into the concept of periods, exploring their structure, significance, and how they relate to the electronic configuration and properties of elements.
Understanding Periods: A Horizontal Journey Through Atomic Structure
The horizontal rows in the periodic table are called periods. Each period represents a principal energy level or shell in an atom's electron cloud. As we move across a period from left to right, we sequentially add electrons to the same principal energy level. This gradual addition of electrons significantly influences the chemical and physical properties of the elements within that period. The number of elements in each period varies, dictated by the number of orbitals within each principal energy level.
The Significance of Principal Energy Levels
The principal energy levels, or shells, are regions around the nucleus where electrons are most likely to be found. They are designated by the principal quantum number (n), starting with n=1 for the first energy level, closest to the nucleus. As 'n' increases, the energy level's distance from the nucleus increases, and its capacity to hold electrons expands.
-
Period 1 (n=1): This shortest period contains only two elements, hydrogen (H) and helium (He). They occupy the 1s orbital, which can hold a maximum of two electrons.
-
Period 2 (n=2): This period features eight elements, from lithium (Li) to neon (Ne). Electrons are added to the 2s and 2p orbitals, expanding the electron configuration possibilities.
-
Period 3 (n=3): Similar to Period 2, Period 3 also contains eight elements, sodium (Na) to argon (Ar). Here, electrons populate the 3s and 3p orbitals.
-
Periods 4 and 5 (n=4 and n=5): These periods are longer, accommodating 18 elements each. This increase in length is due to the inclusion of d-orbitals (which can hold up to 10 electrons) in addition to s and p orbitals.
-
Periods 6 and 7 (n=6 and n=7): These periods are even longer, containing 32 elements each. This significant increase is attributed to the addition of f-orbitals (which can hold up to 14 electrons), contributing to the complexity of the electron configurations of the elements within these periods.
Trends Across a Period: Observing Patterns in Properties
As we traverse a period, we observe systematic changes in the chemical and physical properties of the elements. These trends are primarily dictated by the increasing nuclear charge and the addition of electrons to the same principal energy level.
Atomic Radius: A Decreasing Trend
The atomic radius, the distance from the nucleus to the outermost electrons, generally decreases across a period. This is because the increasing nuclear charge attracts the outermost electrons more strongly, pulling them closer to the nucleus. While additional electrons are added to the same energy level, the increased positive charge outweighs the electron-electron repulsion, leading to a smaller atomic radius.
Ionization Energy: An Increasing Trend
Ionization energy is the energy required to remove an electron from a gaseous atom. Across a period, ionization energy generally increases. This is a direct consequence of the stronger nuclear attraction experienced by electrons in atoms with higher nuclear charges. Removing an electron becomes increasingly difficult as we move across a period.
Electronegativity: A Growing Tendency
Electronegativity measures an atom's ability to attract electrons in a chemical bond. Electronegativity increases across a period. Atoms with higher nuclear charges are more effective at attracting shared electrons in a covalent bond, thus exhibiting higher electronegativity.
Metallic Character: A Shifting Landscape
Metallic character, representing the tendency of an element to lose electrons and form positive ions, generally decreases across a period. As we move from left to right, elements become less metallic and more non-metallic. This is because the increasing nuclear charge makes it more difficult for atoms to lose electrons.
The Significance of Periodicity: Understanding Repeating Patterns
The periodic table's beauty lies in its ability to showcase periodicity – the recurring patterns in the properties of elements. These trends are not arbitrary; they reflect the underlying structure of atoms and how their electron configurations influence their chemical behavior. Understanding periodicity enables us to predict the properties of elements based on their position within the periodic table, significantly advancing our ability to understand chemical reactions and predict the formation of compounds.
Predicting Chemical Behavior
The trends observed across periods allow chemists to predict how elements will react. For example, elements on the left side of a period (alkali and alkaline earth metals) tend to readily lose electrons, forming positive ions and exhibiting high reactivity. Conversely, elements on the right side (halogens and noble gases) tend to gain electrons or remain unreactive, influencing their bonding behavior.
Designing New Materials
Knowledge of periodic trends is fundamental in materials science. By strategically choosing elements from specific periods, researchers can design materials with desired properties. For instance, understanding the relationship between electronegativity and bond strength allows for the development of stronger or more stable materials.
Beyond the Basics: Delving Deeper into Periodicity
The simple trends outlined above provide a basic understanding of periodicity. However, subtle variations and exceptions exist due to the complexities of electron-electron interactions and shielding effects.
Shielding Effects: Inner Electrons' Influence
Inner electrons shield the outermost electrons from the full effect of the nuclear charge. This shielding effect modifies the trends observed across periods, particularly in elements with multiple electron shells. Heavier elements often exhibit less pronounced trends due to the increased shielding effects.
Electron Configurations and Exceptions
Although electron configurations generally follow predictable patterns, exceptions exist. Certain elements exhibit anomalous electron configurations due to the relative energies of orbitals and the stability associated with half-filled or fully filled subshells. These exceptions subtly affect the trends across periods.
Practical Applications: The Impact of Periodicity in Everyday Life
The understanding of periods and their related trends is not confined to theoretical chemistry. It has widespread applications in various fields.
Understanding Chemical Reactions
The reactivity of elements, governed by their position in the periodic table, is crucial for understanding chemical reactions. This knowledge is fundamental in diverse fields, from industrial chemical processes to designing efficient and safe pharmaceuticals.
Developing New Technologies
The development of new materials with specific properties relies heavily on an understanding of periodic trends. This is particularly relevant in advanced technologies such as nanotechnology, where the properties of materials at the atomic scale are crucial.
Environmental Science
Understanding the behavior of elements is fundamental for environmental science, including assessing the impact of pollutants and developing strategies for environmental remediation. The properties of elements, governed by their position in the periodic table, are crucial for understanding their interactions with the environment.
Conclusion: The Period Table – A Map to Chemical Understanding
The horizontal rows, or periods, in the periodic table are not merely a numbering system; they are fundamental to understanding the underlying structure and properties of elements. By exploring the trends across periods, we gain insights into atomic radii, ionization energies, electronegativities, and metallic character, leading to a deeper understanding of chemical behavior and reactivity. The knowledge gained from studying periods is invaluable in various fields, underpinning technological advancements, chemical synthesis, and environmental science. The periodic table, with its elegantly organized periods and groups, remains an indispensable tool for chemists and scientists across disciplines. Mastering the concept of periods is essential for any aspiring chemist or anyone seeking a deeper understanding of the material world around us.
Latest Posts
Latest Posts
-
How To Draw An Integral Sign
Mar 26, 2025
-
Which Plane Divides The Body Into Superior And Inferior Sections
Mar 26, 2025
-
Difference Between Substrate Level Phosphorylation And Oxidative Phosphorylation
Mar 26, 2025
-
5 Steps Of The Listening Process
Mar 26, 2025
-
How To Determine If A Transformation Is Linear
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Horizontal Rows On The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
