What Is The Molecular Ion Peak
Muz Play
Mar 29, 2025 · 7 min read
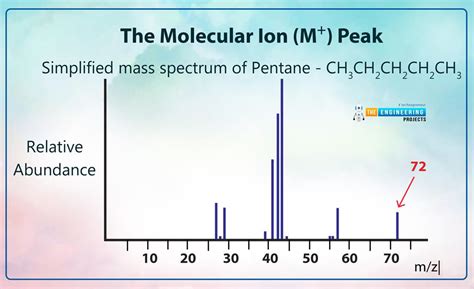
Table of Contents
What is the Molecular Ion Peak? A Comprehensive Guide to Mass Spectrometry
Mass spectrometry (MS) is a powerful analytical technique used to identify and quantify the components of a sample. A crucial aspect of interpreting mass spectrometry data is understanding the molecular ion peak. This peak provides fundamental information about the molecular weight and structure of the analyte. This comprehensive guide will delve into the intricacies of the molecular ion peak, explaining its formation, significance, and limitations, while also exploring related concepts.
Understanding the Basics of Mass Spectrometry
Before we dive into the molecular ion peak, let's briefly review the principles of mass spectrometry. MS works by ionizing a sample, separating the ions based on their mass-to-charge ratio (m/z), and then detecting the abundance of each ion. This process generates a mass spectrum, a graph that plots the relative abundance of ions against their m/z values.
The sample is first introduced into the mass spectrometer, often through various techniques such as direct insertion, gas chromatography (GC), or liquid chromatography (LC). Ionization is a crucial step where neutral molecules are converted into charged ions. Common ionization methods include electron ionization (EI), chemical ionization (CI), electrospray ionization (ESI), and matrix-assisted laser desorption/ionization (MALDI). Each method has its advantages and disadvantages depending on the sample's properties.
After ionization, the ions are accelerated and passed through a mass analyzer, which separates the ions based on their m/z ratio. Different mass analyzers exist, including quadrupole, time-of-flight (TOF), ion trap, and Orbitrap, each with its unique characteristics regarding mass resolution, sensitivity, and speed. Finally, the separated ions are detected, providing the data for the mass spectrum.
What is the Molecular Ion Peak (M⁺)?
The molecular ion peak, often denoted as M⁺ (for positive ions) or M⁻ (for negative ions), represents the parent ion of the molecule being analyzed. It's the ion formed when a molecule loses one electron during the ionization process, resulting in a net positive charge. Its m/z value directly corresponds to the molecular weight of the intact molecule. This makes it a cornerstone for determining the molecule's molecular formula.
For example: If the molecular ion peak is observed at m/z 100, it suggests that the molecule has a molecular weight of 100 Da (Dalton).
Identifying the molecular ion peak is a critical first step in interpreting a mass spectrum. It provides a fundamental piece of information – the molecular weight – that can be used to narrow down the possible structures of the unknown molecule.
Formation of the Molecular Ion Peak
The formation of the molecular ion peak depends heavily on the ionization method employed. Electron ionization (EI), a hard ionization technique, often produces a prominent molecular ion peak, especially for relatively small, stable molecules. EI involves bombarding the molecule with a high-energy electron beam, causing the ejection of an electron and the formation of a radical cation (M⁺•).
However, in softer ionization techniques such as ESI and MALDI, the molecular ion peak might be less intense or even absent. These techniques produce less fragmentation, often resulting in the observation of [M+H]⁺ (protonated molecule) or [M-H]⁻ (deprotonated molecule) ions rather than the radical cation M⁺•, especially for larger molecules or those prone to fragmentation.
The intensity of the molecular ion peak is also influenced by the molecule's structure and stability. Molecules with strong bonds and a stable structure are more likely to produce a more intense molecular ion peak. Conversely, molecules prone to fragmentation may exhibit a weak or absent molecular ion peak, with more abundant fragment ions.
Significance of the Molecular Ion Peak in Mass Spectrometry
The molecular ion peak plays a pivotal role in several aspects of mass spectrometry analysis:
-
Determining Molecular Weight: The m/z value of the molecular ion peak directly provides the molecule's molecular weight, a crucial parameter for identifying the molecule.
-
Determining Molecular Formula: Combining the molecular weight from the molecular ion peak with other information like the isotopic distribution (discussed later) helps determine the molecular formula.
-
Structure Elucidation: The presence, absence, or intensity of the molecular ion peak can provide clues about the molecule's structure and stability. A weak or absent molecular ion peak might suggest a molecule prone to fragmentation.
-
Quantitative Analysis: In selected ion monitoring (SIM) experiments, the molecular ion peak can be used to quantitatively determine the amount of a specific molecule in a sample.
Isotopic Peaks and their Contribution
Most elements exist as a mixture of isotopes, atoms with the same atomic number but different mass numbers. This isotopic abundance affects the appearance of the mass spectrum. For instance, chlorine has two isotopes, ³⁵Cl and ³⁷Cl, with relative abundances of approximately 75% and 25%, respectively. This means that a molecule containing chlorine will exhibit a characteristic isotopic pattern, with peaks corresponding to the different isotopic combinations.
These isotopic peaks are typically found at m/z values higher than the molecular ion peak, with intensity ratios reflecting the natural isotopic abundances. The pattern of these isotopic peaks provides additional information that can be used to confirm the molecular formula. Analyzing the isotopic pattern is particularly useful for determining the number of certain atoms (e.g., chlorine, bromine) in the molecule.
Fragmentation and Fragment Ion Peaks
When a molecule is ionized, it doesn't always remain intact. Often, it undergoes fragmentation, breaking into smaller ions. These fragment ions produce peaks in the mass spectrum at m/z values lower than the molecular ion peak. Analyzing these fragment ion peaks is crucial for determining the structure of the molecule. The fragmentation pattern is highly characteristic of the molecule's structure and functional groups.
Limitations of Relying Solely on the Molecular Ion Peak
While the molecular ion peak is extremely valuable, relying solely on it can be misleading or insufficient:
-
Weak or Absent Molecular Ion Peak: As previously mentioned, some molecules may produce a weak or absent molecular ion peak, making it challenging to determine the molecular weight. This often occurs with larger molecules or those that readily fragment.
-
Isomeric Molecules: Isomers (molecules with the same molecular formula but different structures) may exhibit similar molecular ion peaks, requiring further analysis of fragmentation patterns to differentiate them.
-
Interference from Impurities: Impurities in the sample can create peaks that overlap with the molecular ion peak, leading to inaccurate determinations of the molecular weight.
-
Ion Suppression: In complex mixtures, some ions might suppress the ionization of others, reducing the intensity of the molecular ion peak and potentially masking its presence.
Advanced Techniques and Strategies
To overcome the limitations associated with relying solely on the molecular ion peak, several advanced techniques and strategies are often employed:
-
Chemical Ionization (CI): CI is a softer ionization technique that produces less fragmentation than EI, often resulting in a more prominent molecular ion peak (or quasi-molecular ions such as [M+H]+).
-
High-Resolution Mass Spectrometry (HRMS): HRMS provides accurate mass measurements, enabling precise determination of the molecular formula and differentiation between isomers with the same nominal mass.
-
Tandem Mass Spectrometry (MS/MS): MS/MS involves selecting a precursor ion (often the molecular ion) and fragmenting it further to obtain more structural information.
-
Data Processing and Interpretation Software: Sophisticated software packages aid in the analysis of mass spectra, including algorithms for peak identification, deconvolution, and structural elucidation.
Conclusion
The molecular ion peak is a critical component in mass spectrometry, providing essential information about a molecule's molecular weight and contributing significantly to structure elucidation. However, it's crucial to understand its limitations and employ various complementary techniques and strategies to achieve accurate and comprehensive results. Mastering the interpretation of the molecular ion peak and associated fragment ions is a fundamental skill for anyone working with mass spectrometry data. Combining this knowledge with advanced techniques and careful consideration of experimental conditions is crucial for robust and reliable analysis. The ability to interpret the intricate details of a mass spectrum, utilizing the molecular ion peak as a starting point, is key to unlocking the secrets of the molecular world.
Latest Posts
Latest Posts
-
Is Urea The Same As Uric Acid
Mar 31, 2025
-
How To Determine The Highest Boiling Point
Mar 31, 2025
-
How To Explain 10x In Lab Math
Mar 31, 2025
-
How To Make A Normal Probability Plot
Mar 31, 2025
-
Introspection Refers To A Process By Which Someone Examines
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molecular Ion Peak . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
