What Is The Property Of Bases
Muz Play
Mar 22, 2025 · 6 min read
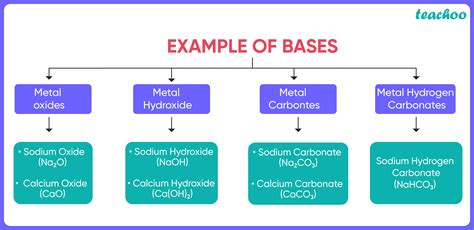
Table of Contents
- What Is The Property Of Bases
- Table of Contents
- What are the Properties of Bases? A Comprehensive Guide
- Defining Bases: Arrhenius, Brønsted-Lowry, and Lewis Definitions
- 1. Arrhenius Definition:
- 2. Brønsted-Lowry Definition:
- 3. Lewis Definition:
- Key Properties of Bases: A Detailed Exploration
- 1. pH Greater than 7:
- 2. Bitter Taste:
- 3. Slippery or Soapy Feel:
- 4. Reaction with Acids:
- 5. Conduct Electricity:
- 6. Change Indicator Color:
- 7. Reactivity with Metals:
- 8. Catalytic Activity:
- Classification of Bases: Strength and Types
- 1. Strength of Bases:
- 2. Types of Bases:
- Applications of Bases: A Wide Range of Uses
- 1. Industrial Applications:
- 2. Everyday Applications:
- Safety Precautions: Handling Bases with Care
- Conclusion: Understanding the Significance of Bases
- Latest Posts
- Latest Posts
- Related Post
What are the Properties of Bases? A Comprehensive Guide
Bases are fundamental chemical compounds with distinct properties that make them crucial in various applications, from everyday household cleaning to complex industrial processes. Understanding these properties is key to appreciating their significance and safe handling. This comprehensive guide delves into the characteristics of bases, exploring their chemical behavior, physical attributes, and practical implications.
Defining Bases: Arrhenius, Brønsted-Lowry, and Lewis Definitions
Before exploring their properties, let's clarify what constitutes a base. Several definitions exist, each offering a slightly different perspective:
1. Arrhenius Definition:
This classic definition, proposed by Svante Arrhenius, defines a base as a substance that increases the concentration of hydroxide ions (OH⁻) when dissolved in water. This increase in OH⁻ ions leads to an increase in the solution's pH, making it alkaline. Examples include sodium hydroxide (NaOH) and potassium hydroxide (KOH), which readily dissociate in water to release OH⁻ ions.
2. Brønsted-Lowry Definition:
Johannes Nicolaus Brønsted and Thomas Martin Lowry independently proposed a broader definition. They defined a base as a proton (H⁺) acceptor. This definition extends beyond aqueous solutions, encompassing reactions in non-aqueous solvents or even in the gas phase. A Brønsted-Lowry base accepts a proton from an acid, forming its conjugate acid. For example, ammonia (NH₃) acts as a base by accepting a proton from water, forming the ammonium ion (NH₄⁺).
3. Lewis Definition:
Gilbert N. Lewis offered the most general definition, encompassing even more types of reactions. A Lewis base is defined as an electron-pair donor. This definition includes substances that don't necessarily contain hydroxide ions or accept protons. A Lewis base donates a lone pair of electrons to a Lewis acid, forming a coordinate covalent bond. For example, ammonia (NH₃) can act as a Lewis base by donating its lone pair of electrons to a metal ion.
These definitions build upon each other, with the Lewis definition being the most inclusive, encompassing all bases defined by the Arrhenius and Brønsted-Lowry definitions.
Key Properties of Bases: A Detailed Exploration
Bases exhibit a range of characteristic properties, many of which are directly related to their ability to accept protons or donate electron pairs.
1. pH Greater than 7:
The most recognizable property of bases is their alkaline nature, resulting in a pH greater than 7 (at 25°C). This indicates a higher concentration of hydroxide ions (OH⁻) compared to hydronium ions (H₃O⁺). The higher the pH, the stronger the base. Strong bases have pH values close to 14, while weak bases have pH values closer to 7.
2. Bitter Taste:
While not a recommended method for identification, bases generally have a bitter taste. This should never be used to test for bases due to potential harm.
3. Slippery or Soapy Feel:
Many bases, especially strong ones, exhibit a slippery or soapy feel when touched. This is because they react with oils and fats on the skin, forming soap-like substances. This property should also not be used as a test; direct contact with strong bases can cause severe skin irritation and burns.
4. Reaction with Acids:
The most fundamental chemical property of a base is its reaction with acids. This reaction, called neutralization, produces water and a salt. The heat released during this reaction is often significant, especially with strong acids and bases. For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) produces sodium chloride (NaCl) and water (H₂O):
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
5. Conduct Electricity:
Aqueous solutions of bases, especially strong bases, conduct electricity. This is because the dissolved base dissociates into ions, which are charge carriers and facilitate the flow of electric current. The strength of the conductivity depends on the base's strength and concentration.
6. Change Indicator Color:
Bases cause distinct color changes in acid-base indicators, such as litmus paper and phenolphthalein. Litmus paper turns blue in the presence of a base, while phenolphthalein turns pink. These indicators are useful for quickly determining the basic nature of a solution.
7. Reactivity with Metals:
Some bases, particularly those of strong alkali metals (like sodium and potassium), can react vigorously with certain metals, such as aluminum and zinc, liberating hydrogen gas. This reaction is often exothermic and should be handled with caution.
8. Catalytic Activity:
Certain bases exhibit catalytic activity, meaning they can accelerate the rate of chemical reactions without being consumed themselves. This property is exploited in various industrial processes, such as the production of biodiesel and certain organic synthesis reactions.
Classification of Bases: Strength and Types
Bases are broadly categorized based on their strength and chemical structure.
1. Strength of Bases:
Bases are classified as strong or weak based on their degree of dissociation in water.
-
Strong Bases: These bases completely dissociate into their ions in aqueous solution. Examples include sodium hydroxide (NaOH), potassium hydroxide (KOH), calcium hydroxide (Ca(OH)₂), and barium hydroxide (Ba(OH)₂).
-
Weak Bases: These bases only partially dissociate in water, resulting in a lower concentration of hydroxide ions. Examples include ammonia (NH₃), pyridine (C₅H₅N), and many organic amines.
2. Types of Bases:
Bases can also be classified based on their chemical composition:
-
Alkali Metal Hydroxides: These are strong bases, like NaOH and KOH, formed by alkali metals reacting with water.
-
Alkaline Earth Metal Hydroxides: These are moderately strong bases, such as Ca(OH)₂ and Mg(OH)₂.
-
Ammonium Hydroxide: While technically not a hydroxide, ammonium hydroxide (NH₄OH) acts as a weak base due to the ammonia's ability to accept protons.
-
Organic Bases: Many organic compounds containing nitrogen, such as amines and amides, act as weak bases.
Applications of Bases: A Wide Range of Uses
The diverse properties of bases make them invaluable across numerous applications:
1. Industrial Applications:
-
Manufacturing: Bases are crucial in various industrial processes, such as the production of soaps, detergents, paper, and textiles.
-
Chemical Synthesis: Bases are widely used as catalysts and reactants in various chemical synthesis reactions.
-
Water Treatment: Bases are used to adjust the pH of water, removing acidity and improving water quality.
-
Metal Refining: Certain bases play roles in refining and purifying metals.
2. Everyday Applications:
-
Cleaning Products: Many household cleaning products contain bases, such as oven cleaners and drain cleaners, leveraging their ability to dissolve grease and other substances.
-
Food Industry: Certain bases are used as food additives, such as baking soda (sodium bicarbonate), which acts as a leavening agent.
-
Agriculture: Bases are sometimes used to adjust soil pH, making it suitable for plant growth.
-
Medicine: Some bases are used in medications, either directly or as components in drug formulations.
Safety Precautions: Handling Bases with Care
Strong bases are corrosive and can cause severe damage to skin, eyes, and other tissues. Always handle bases with appropriate safety precautions:
-
Wear protective gear: This includes gloves, goggles, and lab coats.
-
Work in a well-ventilated area: Some bases produce noxious fumes.
-
Handle with care: Avoid spills and direct contact with skin or eyes.
-
Neutralize spills promptly: Use a suitable acid to neutralize any spills, following proper safety procedures.
-
Proper storage: Store bases in appropriate containers in designated areas.
Conclusion: Understanding the Significance of Bases
Bases are integral to our world, underpinning numerous industrial and domestic processes. Their diverse properties, ranging from their ability to neutralize acids to their catalytic activity, are fundamental to their widespread use. By understanding their characteristics and handling them safely, we can harness their potential while minimizing potential risks. This comprehensive guide serves as a foundation for further exploration of these remarkable chemical compounds and their essential roles in various fields.
Latest Posts
Latest Posts
-
What Is The Social Construction Of Race
Mar 24, 2025
-
Experiment 10 Composition Of Potassium Chlorate
Mar 24, 2025
-
Capacitance Of A Parallel Plate Capacitor With Dielectric Slab
Mar 24, 2025
-
What Is A Power Stroke During Muscle Contraction
Mar 24, 2025
-
Enter The Assignment Of The Observed Transition Violet
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about What Is The Property Of Bases . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
