What Makes Up Most Of The Mass Of An Atom
Muz Play
Mar 19, 2025 · 5 min read
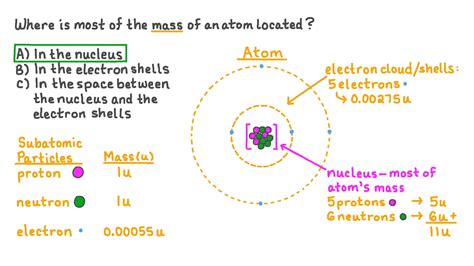
Table of Contents
What Makes Up Most of the Mass of an Atom?
Understanding the composition of an atom is fundamental to grasping the principles of chemistry and physics. While atoms are incredibly tiny, they are far from simple. They consist of various subatomic particles, each playing a crucial role in determining the atom's properties. But the question remains: what constitutes the bulk of an atom's mass? The answer lies primarily within the atom's nucleus.
The Atom's Nucleus: The Mass Heavyweight Champion
The atom's nucleus is a dense, central region containing two types of particles: protons and neutrons. These particles are collectively known as nucleons. It's the nucleons, and particularly the protons and neutrons, that contribute the vast majority of an atom's mass.
Protons: Positive Charge and Significant Mass
Protons carry a positive electrical charge, and their number defines the atomic number of an element. This number determines the element's identity—an atom with one proton is hydrogen, two protons is helium, and so on. Importantly, protons contribute significantly to the atom's overall mass. A single proton has a mass of approximately 1.6726 x 10^-27 kilograms.
Neutrons: Neutral Charge, Significant Mass
Neutrons, as their name suggests, carry no electrical charge. They are electrically neutral. However, their mass is very similar to that of a proton, approximately 1.6749 x 10^-27 kilograms. The presence of neutrons within the nucleus plays a crucial role in stabilizing the nucleus, preventing the electrostatic repulsion between positively charged protons from causing the nucleus to break apart. The number of neutrons in an atom can vary, even for the same element, resulting in isotopes.
Electrons: Negligible Mass, Significant Role
Electrons are negatively charged particles that orbit the atom's nucleus. They occupy regions of space called orbitals, which are defined by quantum mechanics. Unlike protons and neutrons residing in the nucleus, electrons are found in the atom's electron cloud. This cloud is far larger than the nucleus itself, but the electron's mass is significantly less than that of protons and neutrons.
The mass of an electron is approximately 9.1094 x 10^-31 kilograms – almost 2000 times less massive than a proton or neutron. While electrons are crucial for chemical bonding and determining an atom's chemical properties, their contribution to the overall mass of the atom is negligible.
The Mass Number: A Convenient Metric
The mass number of an atom is the total number of protons and neutrons in its nucleus. It's represented as a superscript to the left of the element's symbol (e.g., ¹²C for carbon-12). The mass number provides a convenient way to approximate the atom's mass, although it doesn't directly account for the minute mass of electrons. Because the mass of electrons is so small, we typically ignore it when calculating atomic mass.
Isotopes and Atomic Mass
Elements can exist as different isotopes, which are atoms of the same element with the same number of protons but differing numbers of neutrons. For example, carbon-12 (¹²C) has six protons and six neutrons, while carbon-14 (¹⁴C) has six protons and eight neutrons. Both are isotopes of carbon, but they differ in mass due to the extra neutrons in ¹⁴C.
The atomic mass (or atomic weight) of an element is a weighted average of the masses of its naturally occurring isotopes. This takes into account the relative abundance of each isotope. For instance, the atomic mass of carbon is approximately 12.011 atomic mass units (amu), slightly higher than the mass of ¹²C because of the presence of heavier isotopes like ¹³C and ¹⁴C.
The Significance of Nuclear Mass
The dominance of nuclear mass (protons and neutrons) in determining the overall mass of an atom has profound implications in various fields:
- Nuclear Physics: Understanding the strong nuclear force that binds protons and neutrons together in the nucleus is crucial for studying nuclear reactions, such as fission and fusion. These reactions involve tremendous energy changes due to the significant mass changes associated with the rearrangement of nucleons.
- Chemistry: Although electron configuration determines chemical reactivity, the mass of the atom (largely determined by the nucleus) influences physical properties like density and diffusion rates. Heavier atoms will generally have a higher density than lighter atoms.
- Cosmology and Astrophysics: The mass of atoms is a key factor in understanding the formation of stars and galaxies. Nuclear fusion in stars converts mass into energy, driving stellar processes. The abundance of various elements in the universe is directly linked to nuclear reactions that have occurred over cosmic time.
Beyond Protons and Neutrons: A Deeper Dive
While protons and neutrons are the primary contributors to an atom's mass, they are themselves composite particles. They are made up of even smaller fundamental particles called quarks. Quarks are bound together by the strong force, mediated by gluons. However, the mass of the quarks themselves only accounts for a small fraction of the proton and neutron's mass.
A significant portion of the proton and neutron's mass arises from the energy associated with the strong force that binds the quarks together, a manifestation of Einstein's famous equation, E=mc². This energy contributes substantially to the overall mass of the nucleons and, consequently, the atom.
Conclusion: A Simplified Overview
In summary, the bulk of an atom's mass resides within its nucleus, specifically in the protons and neutrons. Although electrons are essential for chemical behavior and determine an atom's charge, their contribution to its overall mass is practically negligible. The mass number provides a convenient approximation of the atom's mass, while the atomic mass accounts for the presence of different isotopes. Understanding the mass composition of an atom is crucial for diverse fields, ranging from nuclear physics and chemistry to cosmology and astrophysics. The seemingly simple atom reveals incredible complexity when delving into the fundamental particles and forces that govern its structure and behavior. The contribution of the strong force energy to the mass of protons and neutrons adds another layer to this intricate puzzle, highlighting the fascinating interplay between mass and energy within the atomic world.
Latest Posts
Latest Posts
-
Use Inverse Matrix To Solve System Of Equations
Mar 19, 2025
-
New Research Indicates Changes In The Teenage Brain What Occurs
Mar 19, 2025
-
Where Does Fermentation Occur In A Cell
Mar 19, 2025
-
Work In A N Electric Field
Mar 19, 2025
-
Introduction To Acids And Bases Worksheet
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Makes Up Most Of The Mass Of An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
