What Reagent Is Used To Test For Starch
Muz Play
Mar 27, 2025 · 5 min read
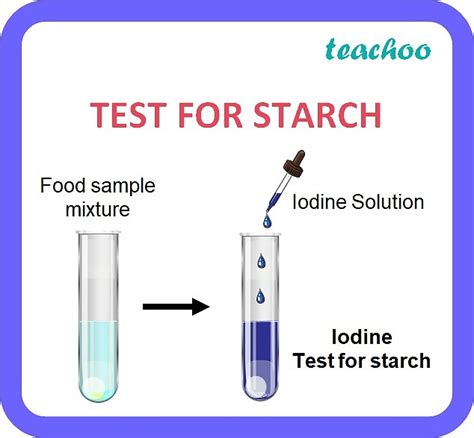
Table of Contents
What Reagent is Used to Test for Starch? A Deep Dive into Iodine and its Applications
Starch, a ubiquitous carbohydrate in our diets and a crucial component of many industrial processes, is easily identifiable through a simple yet elegant chemical test. This article delves deep into the reagent used to test for starch: iodine, exploring its chemical properties, the mechanism behind the color change reaction, applications beyond starch detection, and considerations for accurate testing.
Understanding Iodine and its Interaction with Starch
The reagent of choice for starch detection is a solution of iodine, typically in the form of iodine potassium iodide (IKI). This is because elemental iodine (I₂) is not very soluble in water, but adding potassium iodide (KI) significantly increases its solubility, creating a readily usable solution. The solution's color is typically a brownish-yellow.
The Chemistry of the Starch-Iodine Reaction
The reaction between iodine and starch is a classic example of a complexation reaction, not a chemical reaction in the strictest sense. Iodine doesn't chemically react with starch; instead, the iodine molecules are trapped within the helical structure of the amylose component of starch.
Amylose, a linear polysaccharide, forms a helix. The iodine molecules fit snugly inside these helical cavities, forming a charge-transfer complex. This complex absorbs light differently than free iodine molecules, leading to a dramatic color change. The size and shape of the amylose helix are crucial for the reaction's effectiveness; other polysaccharides, lacking a similar structure, don't exhibit the same color change.
The Characteristic Color Change: A Visual Indicator
The most striking aspect of the starch-iodine test is the intense blue-black color that develops upon the addition of the iodine solution to a starch solution. This vivid color change serves as a clear visual indicator of the presence of starch. The intensity of the color is directly proportional to the amount of starch present; a higher concentration of starch results in a darker blue-black color.
Conversely, the absence of a color change indicates the absence of starch. This simple observation is the basis for the widespread use of the iodine test in various applications.
Practical Applications of the Starch-Iodine Test
The simplicity and reliability of the starch-iodine test make it incredibly versatile, finding applications in diverse fields.
Food Science and Nutrition
The test is routinely used in food science and nutrition to:
- Detect the presence of starch in food samples: This is crucial for quality control, labeling accuracy, and nutritional analysis. From bread and pasta to fruits and vegetables, the iodine test helps determine starch content.
- Identify adulteration in food products: The test can detect the presence of cheaper, starchy fillers in products where starch isn't expected, ensuring food authenticity.
- Monitor the starch content during food processing: This is especially important in industries producing starch-based products like syrups and sweeteners, ensuring consistent product quality.
Biology and Education
In biological laboratories and educational settings, the starch-iodine test is a staple:
- Demonstrating photosynthesis: The test is used to visualize starch production in plants after exposure to light, providing a clear demonstration of photosynthetic processes.
- Studying enzyme activity: Amylase, an enzyme that breaks down starch, can be studied using the iodine test to monitor the rate of starch degradation. The disappearance of the blue-black color signifies enzymatic activity.
- Simple and educational laboratory experiments: The test is a great way to introduce students to basic chemical tests and the concept of qualitative analysis. The immediate visual result makes it engaging and easy to understand.
Other Industrial Applications
Beyond food science and biology, the starch-iodine test finds use in:
- Textile industry: The test can be used to assess the starch content in fabrics, ensuring proper finishing and quality control.
- Paper industry: Starch is used as an adhesive in paper production. The test can monitor the starch concentration during manufacturing.
- Pharmaceutical industry: Starch is used as an excipient in many pharmaceutical formulations. The test helps maintain quality and consistency.
Factors Affecting the Accuracy of the Starch-Iodine Test
While the starch-iodine test is generally reliable, several factors can influence its accuracy:
- Concentration of iodine solution: Using a solution that is too dilute may result in a weak color change, while one that is too concentrated might mask the presence of small amounts of starch. Optimizing the iodine solution concentration is crucial for reliable results.
- Temperature: The intensity of the blue-black color can be affected by temperature. Generally, higher temperatures can weaken the color intensity. Maintaining consistent temperature is important for comparable results.
- Presence of interfering substances: Other substances in the sample can potentially interfere with the test. Reducing agents can react with iodine, affecting the color change. Proper sample preparation and purification may be necessary to eliminate interference.
- Type of starch: Different types of starch, like amylose and amylopectin, react slightly differently with iodine. Amylose gives a more intense blue-black color compared to amylopectin which gives a reddish-brown color. Understanding these differences is crucial for interpreting the results.
Variations and Alternatives to the Iodine Test
While the IKI test is the most common method, some variations and alternative methods exist for detecting starch:
- Using different iodine solutions: Other iodine-based solutions, like Lugol's solution, can also be used, though the composition might slightly affect the sensitivity of the test.
- Using other dyes: Although less common, some other dyes can show a color change in the presence of starch, offering alternative methods, but generally IKI remains the gold standard.
Conclusion: A Powerful and Versatile Test
The starch-iodine test, utilizing iodine potassium iodide (IKI) solution, remains a cornerstone technique in various fields. Its simplicity, sensitivity, and immediate visual readout make it an indispensable tool for detecting the presence and quantifying the amount of starch in diverse samples. Understanding the chemical principles, practical applications, and potential limitations of the test is crucial for its effective and accurate use. By optimizing conditions and being aware of potential interfering factors, researchers and practitioners can confidently rely on the starch-iodine test for accurate and reliable results. Its continued relevance underscores its enduring value as a simple yet powerful analytical tool.
Latest Posts
Latest Posts
-
What Is Found In Animal Cells But Not Plant
Mar 30, 2025
-
Fruit With Orange Flesh And A Large Pit
Mar 30, 2025
-
How Is Commitment Defined By Marcia
Mar 30, 2025
-
Exponential And Logarithmic Equations Quick Check
Mar 30, 2025
-
Charging And Discharging Of Capacitor Formula
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Reagent Is Used To Test For Starch . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
