What Sublevels Are Filling Across The Transition Elements
Muz Play
Mar 27, 2025 · 6 min read
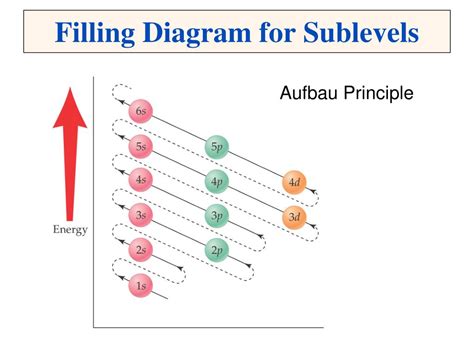
Table of Contents
What Sublevels are Filling Across the Transition Elements?
The transition elements, also known as transition metals, represent a fascinating chapter in the periodic table. Their unique properties stem from the complex ways their electrons are arranged, specifically the filling of their d and, in some cases, f sublevels. Understanding this electron configuration is key to comprehending their diverse chemical and physical behaviors. This article delves deep into the intricacies of sublevel filling across these elements, exploring the exceptions and nuances that make them so intriguing.
The Basics: Electron Configuration and the Aufbau Principle
Before diving into the specifics of transition elements, let's refresh the fundamentals. The Aufbau principle dictates that electrons fill atomic orbitals in order of increasing energy. This generally follows the pattern 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, and so on. This seemingly straightforward rule, however, encounters complexities when dealing with the transition elements.
The key players here are the s, p, and d orbitals. The s orbital can hold a maximum of two electrons, the p orbital can hold six, and the d orbital can hold ten. The filling of these orbitals determines the element's chemical properties and its position within the periodic table.
The Transition Elements: Filling the d Sublevel
The transition elements are characterized by the partial filling of their d electron subshells. This means that their outermost electron shell is not fully filled, which is why their chemistry is so rich and varied. They're found in the d-block of the periodic table, spanning groups 3 to 12.
Let's examine how the d sublevel fills across the periods:
-
First-row transition elements (3d series): These elements, from Scandium (Sc) to Zinc (Zn), are filling their 3d orbitals. The 4s orbital fills before the 3d orbital, resulting in configurations where the 4s orbital is filled first (with two electrons), and then the 3d orbitals fill gradually. However, there are exceptions and subtle variations in the filling order.
-
Second-row transition elements (4d series): Similar to the first row, the 5s orbital fills before the 4d orbitals. These elements exhibit similar trends in electronic configuration but with subtle differences due to the increasing nuclear charge.
-
Third-row transition elements (5d series): The 6s orbital fills prior to the 5d orbitals. The increasing size of the atom and the shielding effect of the inner electrons influence the energy levels and filling order.
-
Fourth-row transition elements (6d series – incomplete): This series includes the actinides, which exhibit even more complex filling patterns due to the interplay of 5f and 6d orbitals. The filling order is further complicated by relativistic effects influencing the energies of the orbitals.
Exceptions to the Rules: The Unexpected Behavior
The Aufbau principle, while a useful guideline, isn't an absolute law. Several exceptions arise within the transition elements, primarily due to the small energy differences between the (n-1)d and ns orbitals. These small energy differences can lead to electron configurations that deviate from the expected filling order. For instance:
-
Chromium (Cr): Instead of the expected [Ar] 3d<sup>4</sup>4s<sup>2</sup> configuration, chromium has a [Ar] 3d<sup>5</sup>4s<sup>1</sup> configuration. This half-filled 3d subshell provides extra stability due to exchange energy.
-
Copper (Cu): Copper deviates from the expected [Ar] 3d<sup>9</sup>4s<sup>2</sup> configuration to a more stable [Ar] 3d<sup>10</sup>4s<sup>1</sup> configuration. A completely filled 3d subshell provides greater stability.
These exceptions highlight the importance of considering the energy stabilization effects of half-filled and fully filled subshells. The energy gained from these configurations outweighs the energy cost of deviating from the strict Aufbau principle.
The Influence of the d Orbitals: Properties of Transition Elements
The filling of the d orbitals profoundly influences the properties of transition elements:
-
Variable Oxidation States: The relatively small energy differences between the d orbitals and the outermost s orbitals allow for the loss of electrons from both subshells, resulting in multiple oxidation states. This versatility is a hallmark of transition metal chemistry and is responsible for their participation in a wide variety of chemical reactions.
-
Colored Compounds: The d orbitals are involved in the absorption and emission of light, causing many transition metal compounds to exhibit vibrant colors. The specific color depends on the oxidation state, ligands (atoms or molecules bonded to the central metal ion), and geometry of the complex.
-
Catalytic Activity: The ability of transition metals to readily accept and donate electrons makes them excellent catalysts. They can form intermediate complexes with reactants, lowering the activation energy and speeding up chemical reactions. Many industrial processes rely on transition metal catalysts.
-
Magnetic Properties: The presence of unpaired electrons in the d orbitals gives many transition metals paramagnetic properties – they are attracted to magnetic fields. Some transition metal compounds exhibit ferromagnetism (strong permanent magnetism).
-
Alloy Formation: Transition elements readily form alloys with each other and other metals. Alloys often have properties superior to those of the constituent metals.
Beyond the d Orbitals: The f-Block Elements (Inner Transition Elements)
Moving beyond the d-block, we encounter the inner transition elements: the lanthanides and actinides. These elements are characterized by the filling of the f orbitals. These elements are less common and often radioactive.
-
Lanthanides (4f series): The 4f orbitals fill across the lanthanides, influencing their chemical behavior and resulting in a greater similarity in properties among them compared to the transition metals. Their chemistry is dominated by the +3 oxidation state.
-
Actinides (5f series): The actinides show a wider range of oxidation states than the lanthanides, due to the relatively closer energy levels of the 5f, 6d, and 7s orbitals. Many actinides are radioactive and exhibit complex chemistry.
Conclusion: A Complex and Fascinating Area of Chemistry
The filling of sublevels across the transition elements is a complex process with numerous exceptions and nuances. Understanding the interplay between the s and d orbitals, and in the case of the inner transition elements, the f orbitals, is crucial for grasping the unique chemical and physical properties of these elements. Their varied oxidation states, catalytic abilities, colorful compounds, and magnetic properties are all consequences of this intricate electronic structure. While the Aufbau principle provides a starting point, exceptions highlight the importance of considering factors like electron-electron repulsion and the stability associated with half-filled and fully filled sublevels. The transition elements continue to be a rich area of research, presenting ongoing challenges and discoveries in the field of chemistry.
Latest Posts
Latest Posts
-
Oral Care For An Unconscious Patient
Mar 30, 2025
-
Solving Linear Equations With Two Unknowns
Mar 30, 2025
-
Equation Of Curve Of Best Fit
Mar 30, 2025
-
Right Handed Vs Left Handed Helix
Mar 30, 2025
-
What Organisms Obtain Its Food From Other Organisms
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Sublevels Are Filling Across The Transition Elements . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
