When Do You Use Henderson Hasselbalch Equation
Muz Play
Mar 30, 2025 · 6 min read
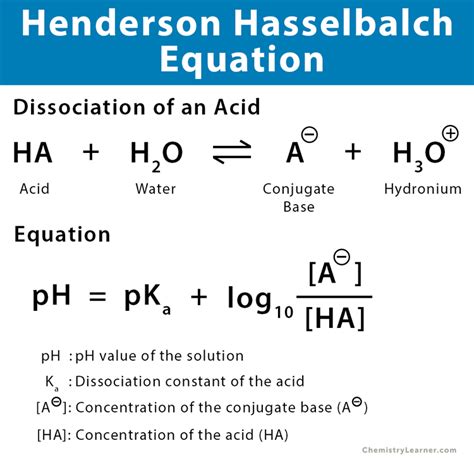
Table of Contents
When Do You Use the Henderson-Hasselbalch Equation? A Comprehensive Guide
The Henderson-Hasselbalch equation is a cornerstone in chemistry, particularly in the fields of biochemistry, pharmacology, and environmental science. It provides a simple yet powerful tool for calculating the pH of a buffer solution, understanding acid-base equilibria, and predicting the behavior of weak acids and bases. However, its application isn't universally applicable, and understanding its limitations is just as crucial as understanding its strengths. This comprehensive guide delves into the intricacies of the Henderson-Hasselbalch equation, exploring when it's appropriate to use it, when it's not, and its various applications.
Understanding the Henderson-Hasselbalch Equation
Before diving into its applications, let's refresh our understanding of the equation itself:
pH = pKa + log([A⁻]/[HA])
Where:
- pH: The measure of hydrogen ion concentration, indicating the acidity or alkalinity of the solution.
- pKa: The negative logarithm of the acid dissociation constant (Ka) of the weak acid. The pKa is a measure of the acid's strength; a lower pKa indicates a stronger acid.
- [A⁻]: The concentration of the conjugate base.
- [HA]: The concentration of the weak acid.
When to Use the Henderson-Hasselbalch Equation: Key Scenarios
The Henderson-Hasselbalch equation shines in situations involving weak acids and their conjugate bases, specifically within buffer solutions. Here are some key scenarios where its application is particularly valuable:
1. Calculating the pH of a Buffer Solution
This is arguably the most common application. Buffer solutions resist changes in pH upon the addition of small amounts of acid or base. They are crucial in many biological systems and chemical processes. The Henderson-Hasselbalch equation allows for straightforward calculation of the pH of a buffer solution given the pKa of the weak acid and the concentrations of the acid and its conjugate base. For example, it's extensively used in biochemistry to determine the pH of physiological buffers like phosphate buffers.
Example: A buffer solution is prepared using 0.1 M acetic acid (pKa = 4.76) and 0.2 M sodium acetate. Using the Henderson-Hasselbalch equation, the pH of the buffer can be readily calculated.
2. Determining the Ratio of Acid to Conjugate Base for a Desired pH
Often, you might need a buffer solution at a specific pH. The Henderson-Hasselbalch equation allows you to determine the required ratio of weak acid to conjugate base to achieve this target pH. This is crucial in many laboratory settings and industrial applications where precise pH control is vital. For instance, in enzyme assays, the optimal pH for enzyme activity needs to be carefully maintained using buffers prepared with calculated ratios.
3. Understanding Titration Curves
Titration curves visually represent the change in pH of a solution as a titrant (acid or base) is added. The Henderson-Hasselbalch equation helps in interpreting the buffer region of the titration curve, where the pH changes relatively slowly. This region is particularly important for understanding the buffering capacity of the solution. The midpoint of the buffer region corresponds to the pKa of the weak acid, providing a valuable experimental way to determine pKa values.
4. Predicting Drug Absorption and Distribution
In pharmacology, the Henderson-Hasselbalch equation is vital in understanding drug absorption and distribution in the body. Many drugs are weak acids or bases, and their ionization state (which depends on the pH of the environment) influences their ability to cross biological membranes. For example, a weak acid drug will be more readily absorbed from the stomach (low pH) than from the intestine (higher pH) because it is predominantly in its non-ionized, lipid-soluble form in the acidic environment.
5. Environmental Chemistry Applications
The equation finds applications in environmental studies, helping in understanding acid-base equilibria in natural water systems like lakes and rivers. The pH of these systems impacts the solubility and bioavailability of various pollutants and nutrients. The Henderson-Hasselbalch equation helps model these systems and predict the environmental fate of chemicals.
When NOT to Use the Henderson-Hasselbalch Equation: Limitations and Cautions
Despite its usefulness, the Henderson-Hasselbalch equation has limitations. It's crucial to understand these limitations to avoid erroneous results:
1. Strong Acids and Bases
The equation is specifically designed for weak acids and bases. Applying it to strong acids or bases will yield inaccurate results because strong acids and bases completely dissociate in solution, invalidating the assumptions underlying the equation.
2. Highly Concentrated Solutions
The equation assumes ideal behavior, which means that the activity coefficients of the acid and its conjugate base are approximately equal to 1. This assumption breaks down in highly concentrated solutions where intermolecular interactions become significant. In such cases, activity coefficients must be considered for accurate calculations, often requiring more complex thermodynamic approaches.
3. Solutions with Significant Ionic Strength
High ionic strength can alter the activity coefficients of the acid and its conjugate base, affecting the accuracy of the calculations. In solutions with high ionic strength, activity corrections are necessary, which goes beyond the scope of the simple Henderson-Hasselbalch equation.
4. Cases Where [A⁻] or [HA] are Very Small or Zero
If the concentration of either the acid or the conjugate base is extremely low or zero, the log term in the Henderson-Hasselbalch equation becomes undefined or very large, leading to inaccurate pH predictions. In such cases, a more precise approach is needed, such as using the full equilibrium expression for the acid dissociation.
5. Polyprotic Acids
The Henderson-Hasselbalch equation is strictly applicable to monoprotic acids (acids that donate only one proton). Polyprotic acids, which can donate multiple protons, require a more complex approach involving multiple equilibrium expressions for each dissociation step.
Beyond the Basics: Advanced Applications and Considerations
The Henderson-Hasselbalch equation forms the basis for more sophisticated analyses of acid-base equilibria. Here are some advanced applications and considerations:
1. Isoelectric Point Calculations
For amino acids and proteins, the isoelectric point (pI) is the pH at which the net charge is zero. The Henderson-Hasselbalch equation plays a crucial role in calculating the pI based on the pKa values of the ionizable groups in the molecule.
2. Buffer Capacity Calculations
While the Henderson-Hasselbalch equation doesn't directly calculate buffer capacity, it is foundational in understanding the factors that influence it. Buffer capacity is directly related to the concentrations of the acid and conjugate base, which are explicitly used in the equation. A greater concentration of both leads to higher buffer capacity.
3. Enzyme Kinetics and Mechanisms
In enzyme kinetics, many enzyme reactions are pH-dependent. The Henderson-Hasselbalch equation aids in understanding how changes in pH affect enzyme activity by altering the ionization state of the enzyme or its substrate.
4. Understanding Solubility and Precipitation
Many metal hydroxides and salts show pH-dependent solubility. The Henderson-Hasselbalch equation, combined with solubility product constants, helps to understand and predict the conditions under which these compounds will precipitate or dissolve.
Conclusion: Mastering the Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation is a powerful tool for understanding and calculating pH in a variety of scenarios. Its simplicity belies its wide-ranging applications in chemistry, biochemistry, pharmacology, and environmental science. However, it’s crucial to remember its limitations and use it judiciously. By understanding its strengths and weaknesses, you can effectively leverage this equation to solve a range of acid-base problems and gain a deeper understanding of chemical equilibria. Remember always to consider the context and ensure the underlying assumptions are met before applying this valuable equation.
Latest Posts
Latest Posts
-
How To Find A Hamilton Circuit
Apr 01, 2025
-
The Positive Subatomic Particle Is The
Apr 01, 2025
-
Which Characteristic Of A Substance Is Considered A Chemical Property
Apr 01, 2025
-
What Major Change Occurs During Metamorphism Of Limestone To Marble
Apr 01, 2025
-
Do All Living Things Respond To Stimuli
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about When Do You Use Henderson Hasselbalch Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
