Where Are The Neutrons Located In The Atom
Muz Play
Mar 24, 2025 · 6 min read
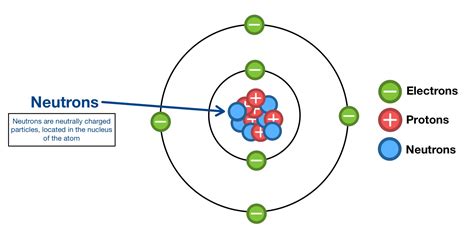
Table of Contents
Where Are the Neutrons Located in the Atom? A Deep Dive into Nuclear Structure
Understanding the location of neutrons within an atom is crucial to comprehending the fundamental nature of matter and the forces that govern the universe. Unlike electrons, which orbit the nucleus in a probabilistic cloud, neutrons reside within the atom's core, the nucleus itself. This seemingly simple statement opens a door to a fascinating world of nuclear physics, exploring the strong nuclear force, isotopic variations, and the implications for nuclear stability and radioactivity. Let's delve into the details.
The Atomic Nucleus: A Dense Core of Protons and Neutrons
The atom, the basic building block of matter, consists of three primary subatomic particles: protons, neutrons, and electrons. Electrons, negatively charged particles, occupy the space surrounding the nucleus in orbitals determined by their energy levels. This electron cloud, though vast compared to the nucleus's size, contributes minimally to the atom's overall mass.
The nucleus, on the other hand, is the atom's dense central core, responsible for almost all of its mass. It houses two types of particles: protons and neutrons. Protons carry a positive electrical charge, while neutrons are electrically neutral, possessing no charge. This seemingly simple difference leads to profound consequences for the atom's properties and behavior. It is within this incredibly dense nucleus that we find our neutrons.
The Strong Nuclear Force: The Glue Holding the Nucleus Together
The nucleus presents a paradox. Protons, being positively charged, should repel each other due to the electromagnetic force, a force that increases dramatically as particles get closer. This repulsion should cause the nucleus to fly apart. Yet, nuclei are stable entities for most atoms. The answer to this conundrum lies in the strong nuclear force.
The strong nuclear force is one of the four fundamental forces of nature, far stronger than the electromagnetic force at short distances. It acts between protons and neutrons within the nucleus, overcoming the electromagnetic repulsion between protons and binding them together. This force is crucial for the stability of the nucleus and thus the existence of atoms beyond hydrogen (which only has a proton and an electron). The neutrons play a vital role in mediating this force, contributing significantly to the nuclear stability.
The Role of Neutrons in Nuclear Stability
The number of protons in an atom's nucleus defines its atomic number and determines its element. However, the number of neutrons can vary, leading to different isotopes of the same element. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. The ratio of protons to neutrons significantly affects the stability of the nucleus.
Isotopic Variations and Nuclear Stability
For lighter elements, a roughly equal number of protons and neutrons often leads to a stable nucleus. As we move to heavier elements, the ratio of neutrons to protons increases to maintain stability. This is because the strong nuclear force has a limited range, and as the number of protons increases, the repulsive electromagnetic force becomes more significant. Adding more neutrons increases the "glue" (strong nuclear force) without adding to the repulsive electromagnetic force, stabilizing the nucleus.
Radioactive Decay: When Nuclei Become Unstable
When the ratio of protons to neutrons deviates too far from the optimal value for stability, the nucleus can become unstable and undergo radioactive decay. This involves the emission of particles or energy to achieve a more stable configuration. Different types of radioactive decay exist, such as alpha decay, beta decay, and gamma decay, each involving the transformation of protons or neutrons. Understanding neutron-proton ratios is fundamental to predicting and understanding radioactive decay processes.
Neutron Distribution Within the Nucleus: A Quantum Mechanical Perspective
While we can say neutrons are located within the nucleus, pinpointing their exact location is impossible due to the principles of quantum mechanics. Unlike classical mechanics, which allows for precise prediction of particle positions and velocities, quantum mechanics describes particles in terms of probabilities.
Quantum Tunneling and the Uncertainty Principle
Neutrons, like all particles, exhibit wave-particle duality. This means they behave as both waves and particles. Their position within the nucleus is not fixed but described by a probability distribution. The uncertainty principle, a cornerstone of quantum mechanics, states that we cannot simultaneously know both the position and momentum of a particle with perfect accuracy. The more precisely we know one, the less precisely we know the other. This inherent uncertainty means we can only talk about the probability of finding a neutron at a particular location within the nucleus.
Nuclear Shell Model: Organizing Neutrons and Protons
The nuclear shell model provides a framework for understanding the arrangement of protons and neutrons within the nucleus. This model suggests that neutrons and protons occupy specific energy levels or shells within the nucleus, much like electrons in an atom. These shells can be filled, and the filling of shells is crucial for determining the stability of the nucleus. A completely filled shell often corresponds to a particularly stable nucleus.
Advanced Concepts: Neutron Stars and Neutron Capture
The importance of neutrons extends far beyond the realm of individual atoms. Their behavior and properties are crucial in understanding larger-scale phenomena.
Neutron Stars: Extreme Density and Neutron Dominance
Neutron stars are among the densest objects in the universe. Formed from the remnants of massive stars that have undergone supernova explosions, these objects are composed primarily of neutrons. The immense gravitational pressure crushes the atoms, forcing electrons and protons to combine into neutrons. Understanding the behavior of neutrons under such extreme conditions is a key area of research in astrophysics.
Neutron Capture: Building Heavier Elements
Neutron capture is a process where an atomic nucleus absorbs a neutron, resulting in an isotope of the same element with one more neutron. This process is crucial in the synthesis of heavier elements in stars. Through a series of neutron captures followed by beta decay, stars create heavier elements, enriching the universe with the building blocks of planets, life, and everything around us.
Conclusion: Understanding Neutrons, Understanding the Universe
The location of neutrons within the atom's nucleus is a fundamental aspect of nuclear physics and has far-reaching implications for our understanding of matter, energy, and the universe. While their precise location is governed by the principles of quantum mechanics and their behavior influences nuclear stability, isotopic variations, and radioactive decay, their importance extends to extreme environments like neutron stars and the creation of heavier elements through neutron capture. Understanding neutrons is not just about understanding atomic structure; it's about understanding the fundamental forces that shape our reality. Further research continues to unveil the intricate complexities and mysteries surrounding these enigmatic particles, continually enriching our comprehension of the universe's workings.
Latest Posts
Latest Posts
-
Definition Of Marketing By Philip Kotler
Mar 28, 2025
-
Formulas For Photosynthesis And Cellular Respiration
Mar 28, 2025
-
The Blank Atom In R 12 Is Believed To Brea Off
Mar 28, 2025
-
Heat Of Neutralization For Hcl And Naoh
Mar 28, 2025
-
Difference Between Selective Media And Differential Media
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Where Are The Neutrons Located In The Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
