Which Change Of State Is Shown In The Model
Muz Play
Mar 28, 2025 · 8 min read
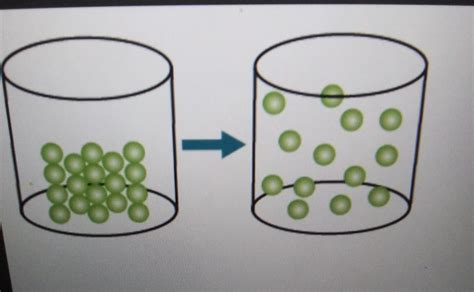
Table of Contents
- Which Change Of State Is Shown In The Model
- Table of Contents
- Which Change of State is Shown in the Model? A Comprehensive Guide
- Understanding the States of Matter
- The Six Changes of State
- 1. Melting (Solid to Liquid)
- 2. Freezing (Liquid to Solid)
- 3. Vaporization (Liquid to Gas)
- 4. Condensation (Gas to Liquid)
- 5. Sublimation (Solid to Gas)
- 6. Deposition (Gas to Solid)
- Identifying the Change of State in a Model
- Examples and Troubleshooting
- Latest Posts
- Latest Posts
- Related Post
Which Change of State is Shown in the Model? A Comprehensive Guide
Understanding changes of state is fundamental to grasping the behavior of matter. This article delves into the various changes of state – melting, freezing, vaporization (including boiling and evaporation), condensation, sublimation, and deposition – and provides a detailed framework for identifying which change is depicted in a given model, whether it's a diagram, a simulation, or a real-world observation. We'll explore the underlying principles, key characteristics, and practical examples to help you confidently identify the phase transition in any scenario.
Understanding the States of Matter
Before diving into the changes, it's crucial to understand the three fundamental states of matter:
-
Solid: Solids have a definite shape and volume. Their particles are tightly packed together in a fixed arrangement, resulting in strong intermolecular forces. Examples include ice, rock, and wood.
-
Liquid: Liquids have a definite volume but take the shape of their container. Their particles are closely packed but can move around relatively freely, resulting in weaker intermolecular forces compared to solids. Examples include water, oil, and mercury.
-
Gas: Gases have neither a definite shape nor volume; they expand to fill their container. Their particles are widely dispersed and move randomly at high speeds, with very weak intermolecular forces. Examples include air, oxygen, and carbon dioxide.
While these are the three primary states, plasma is also considered a state of matter, especially at high temperatures. We won't focus on plasma in this article, as it's less commonly encountered in basic models of phase changes.
The Six Changes of State
The changes of state are transitions between these three primary states. Let's explore each one:
1. Melting (Solid to Liquid)
Melting is the process where a solid transforms into a liquid. This happens when the thermal energy (heat) added overcomes the intermolecular forces holding the solid's particles in a fixed structure. The particles gain enough kinetic energy to break free from their fixed positions and move more freely, leading to the liquid state.
Key Characteristics:
- Absorption of heat: Melting is an endothermic process, meaning it requires energy input.
- Temperature remains constant: During melting, the temperature of the substance remains constant at its melting point until all the solid has melted.
- Increased molecular motion: Particles transition from a rigid structure to a more mobile state.
Example in a Model: A model might show ice cubes gradually transforming into liquid water at 0°C (32°F).
2. Freezing (Liquid to Solid)
Freezing is the reverse of melting. It's the process where a liquid transforms into a solid. This occurs when the thermal energy (heat) is removed from the liquid, causing the particles to lose kinetic energy. The intermolecular forces become dominant, forcing the particles into a fixed, ordered structure.
Key Characteristics:
- Release of heat: Freezing is an exothermic process, meaning it releases energy.
- Temperature remains constant: During freezing, the temperature of the substance remains constant at its freezing point until all the liquid has frozen.
- Decreased molecular motion: Particles transition from a mobile state to a rigid structure.
Example in a Model: A model might depict liquid water turning into ice at 0°C (32°F), showing the formation of a crystalline structure.
3. Vaporization (Liquid to Gas)
Vaporization is the process where a liquid transforms into a gas. This can occur through two primary mechanisms:
-
Boiling: Boiling occurs when a liquid is heated to its boiling point. Bubbles of vapor form throughout the liquid and rise to the surface. The boiling point is the temperature at which the vapor pressure of the liquid equals the external pressure.
-
Evaporation: Evaporation occurs at temperatures below the boiling point. It involves the escape of liquid molecules from the surface of the liquid. Faster-moving molecules near the surface overcome the intermolecular forces and enter the gaseous phase.
Key Characteristics:
- Absorption of heat: Vaporization is an endothermic process, requiring energy input.
- Temperature may or may not remain constant: Boiling occurs at a constant temperature (boiling point), while evaporation can occur over a range of temperatures.
- Increased molecular motion: Particles transition from relatively close proximity to widely dispersed, high-speed motion.
Example in a Model: A model might show water boiling in a pot, with steam rising from the surface, or the gradual disappearance of water from a puddle on a sunny day (evaporation).
4. Condensation (Gas to Liquid)
Condensation is the reverse of vaporization. It's the process where a gas transforms into a liquid. This occurs when the gas cools down, losing kinetic energy. The intermolecular forces become stronger, causing the gas particles to clump together and form liquid droplets.
Key Characteristics:
- Release of heat: Condensation is an exothermic process, releasing energy.
- Temperature may change: Condensation can occur over a range of temperatures.
- Decreased molecular motion: Particles transition from high-speed, random motion to closer proximity with less rapid movement.
Example in a Model: A model might show the formation of dew drops on a cool surface, or water droplets forming on a cold glass of water.
5. Sublimation (Solid to Gas)
Sublimation is the process where a solid directly transforms into a gas without passing through the liquid phase. This occurs when the solid's particles gain enough kinetic energy to overcome the intermolecular forces and escape directly into the gaseous phase.
Key Characteristics:
- Absorption of heat: Sublimation is an endothermic process, requiring energy input.
- Temperature may change: Sublimation can occur over a range of temperatures.
- Significant increase in molecular motion and spacing: Particles transition directly from a rigid, close-packed structure to a widely dispersed, high-speed state.
Example in a Model: A model might depict dry ice (solid carbon dioxide) transforming into carbon dioxide gas at room temperature.
6. Deposition (Gas to Solid)
Deposition is the reverse of sublimation. It's the process where a gas directly transforms into a solid without passing through the liquid phase. This occurs when the gas particles lose sufficient kinetic energy to allow the intermolecular forces to bind them into a solid structure.
Key Characteristics:
- Release of heat: Deposition is an exothermic process, releasing energy.
- Temperature may change: Deposition can occur over a range of temperatures.
- Significant decrease in molecular motion and spacing: Particles transition from a widely dispersed, high-speed state to a rigid, close-packed structure.
Example in a Model: A model might illustrate the formation of frost on a cold surface, where water vapor directly transforms into ice crystals.
Identifying the Change of State in a Model
To identify the change of state shown in a model, carefully observe the following:
-
Initial and final states: Determine the initial and final states of the substance (solid, liquid, or gas).
-
Energy transfer: Determine whether heat is being added (endothermic) or removed (exothermic). Look for indicators like heating elements, cooling surfaces, or changes in temperature.
-
Particle arrangement: If the model shows the arrangement of particles, observe how the arrangement changes during the transition. A transition from a fixed, ordered structure to a more random arrangement suggests melting or sublimation. A transition from a dispersed, random arrangement to a fixed, ordered structure suggests freezing or deposition.
-
Context: Consider the context of the model. What substance is being shown? What are the environmental conditions? This information can provide valuable clues.
Examples and Troubleshooting
Let's consider some scenarios and how to determine the change of state:
Scenario 1: A model shows water in a beaker being heated until it boils and turns into steam.
Analysis: The initial state is liquid (water), the final state is gas (steam), and heat is added. This is vaporization (specifically boiling).
Scenario 2: A model shows water vapor condensing on a cold glass.
Analysis: The initial state is gas (water vapor), the final state is liquid (water), and heat is being removed. This is condensation.
Scenario 3: A model depicts ice crystals forming on a windowpane on a cold winter night.
Analysis: The initial state is gas (water vapor), the final state is solid (ice), and heat is being removed. This is deposition.
Scenario 4: A model illustrates dry ice turning into a gas without forming a liquid phase.
Analysis: The initial state is solid (dry ice), the final state is gas (carbon dioxide), and heat is likely being absorbed (though it might be occurring at room temperature due to dry ice's low sublimation temperature). This is sublimation.
Troubleshooting Common Confusions:
-
Evaporation vs. Boiling: Remember that evaporation occurs below the boiling point and only at the surface, while boiling occurs at the boiling point and throughout the liquid.
-
Sublimation vs. Deposition: Sublimation is solid to gas, while deposition is gas to solid.
By systematically considering the initial and final states, energy transfer, and particle arrangements, you can accurately identify the change of state illustrated in almost any model. Remember to always consult the context and specific details provided within the model for the most accurate determination. With practice, identifying these phase transitions becomes second nature.
Latest Posts
Latest Posts
-
Does Lead Need A Roman Numeral
Apr 01, 2025
-
Introduction To Chemical Reactions Answer Key
Apr 01, 2025
-
Is Melting An Ice Cube A Physical Or Chemical Change
Apr 01, 2025
-
Examples Of A Thesis Statement For A Literary Analysis
Apr 01, 2025
-
What Is The Difference Between Self Esteem And Self Efficacy
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Change Of State Is Shown In The Model . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
